Usage réservé à la recherche
Tox/Tox2 Antibody [H10P3]
N° de cat.: F3505
Application:
Réactivité:
Informations dutilisation
| Dilution |
|---|
|
| Application |
|---|
| WB, IP, IHC |
| Réactivité |
|---|
| Human, Mouse, Rat |
| Source |
|---|
| Rabbit Monoclonal Antibody |
| Tampon de stockage |
|---|
| PBS, pH 7.2+50% Glycerol+0.05% BSA+0.01% NaN3 |
| Stockage (à partir de la date de réception) |
|---|
| -20°C (avoid freeze-thaw cycles), 2 years |
| Poids moléculaire prédit |
|---|
| 60-80 kDa |
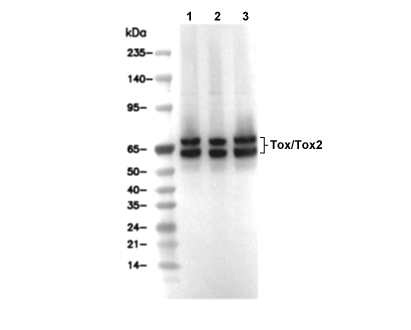
| Contrôle positif | Human spleen; Rat testis; Rat spleen; Rat small intestine; Human tonsil; Human endometrioid adenocarcinoma, Human T-cell lymphoma, Mouse spleen; Human colon carcinoma; Human renal cell carcinoma; Rat thymus; DHL-4 cells; EL4 cells |
|---|---|
| Contrôle négatif | ZR-75-1 cells |
Méthodes expérimentales
| WB |
|---|
Experimental Protocol:
Sample preparation
1. Tissue: Lyse the tissue sample by adding an appropriate volume of ice-cold RIPA/Nuclear Lysis Buffer (containing Protease Inhibitor Cocktail),and homogenize the tissue at a low temperature. 2. Adherent cell: Aspirate the culture medium and wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/Nuclear Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 3. Suspension cell: Transfer the culture medium to a pre-cooled centrifuge tube. Centrifuge and aspirate the supernatant. Wash the cells with ice-cold PBS twice. Lyse the cells by adding an appropriate volume of RIPA/Nuclear Lysis Buffer (containing Protease Inhibitor Cocktail) and put the sample on ice for 5 min. 4. Place the lysate into a pre-cooled microcentrifuge tube. Centrifuge at 4°C for 15 min. Collect the supernatant;
5. Remove a small volume of lysate to determine the protein concentration;
6. Combine the lysate with protein loading buffer. Boil 20 µL sample under 95-100°C for 5 min. Centrifuge for 5 min after cool down on ice.
Electrophoretic separation
1. According to the concentration of extracted protein, load appropriate amount of protein sample and marker onto SDS-PAGE gels for electrophoresis. Recommended separating gel (lower gel) concentration: 10%. Reference Table for Selecting SDS-PAGE Separation Gel Concentrations 2. Power up 80V for 30 minutes. Then the power supply is adjusted (110 V~150 V), the Marker is observed, and the electrophoresis can be stopped when the indicator band of the predyed protein Marker where the protein is located is properly separated. (Note that the current should not be too large when electrophoresis, too large current (more than 150 mA) will cause the temperature to rise, affecting the result of running glue. If high currents cannot be avoided, an ice bath can be used to cool the bath.)
Transfer membrane
1. Take out the converter, soak the clip and consumables in the pre-cooled converter;
2. Activate PVDF membrane with methanol for 1 min and rinse with transfer buffer;
3. Install it in the order of "black edge of clip - sponge - filter paper - filter paper - glue -PVDF membrane - filter paper - filter paper - sponge - white edge of clip"; 4. The protein was electrotransferred to PVDF membrane. ( 0.45 µm PVDF membrane is recommended ) Reference Table for Selecting PVDF Membrane Pore Size Specifications Recommended conditions for wet transfer: 200 mA, 120 min. ( Note that the transfer conditions can be adjusted according to the protein size. For high-molecular-weight proteins, a higher current and longer transfer time are recommended. However, ensure that the transfer tank remains at a low temperature to prevent gel melting.)
Block
1. After electrotransfer, wash the film with TBST at room temperature for 5 minutes;
2. Incubate the film in the blocking solution for 1 hour at room temperature;
3. Wash the film with TBST for 3 times, 5 minutes each time.
Antibody incubation
1. Use 5% skim milk powder to prepare the primary antibody working liquid (recommended dilution ratio for primary antibody 1:1000), gently shake and incubate with the film at 4°C overnight; 2. Wash the film with TBST 3 times, 5 minutes each time;
3. Add the secondary antibody to the blocking solution and incubate with the film gently at room temperature for 1 hour;
4. After incubation, wash the film with TBST 3 times for 5 minutes each time.
Antibody staining
1. Add the prepared ECL luminescent substrate (or select other color developing substrate according to the second antibody) and mix evenly;
2. Incubate with the film for 1 minute, remove excess substrate (keep the film moist), wrap with plastic film, and expose in the imaging system. |
| IHC |
|---|
Experimental Protocol:
Deparaffinization/Rehydration
1. Deparaffinize/hydrate sections:
2. Incubate sections in three washes of xylene for 5 min each.
3. Incubate sections in two washes of 100% ethanol for 10 min each.
4. Incubate sections in two washes of 95% ethanol for 10 min each.
5. Wash sections two times in dH2O for 5 min each.
6.Antigen retrieval: For Citrate: Heat slides in a microwave submersed in 1X citrate unmasking solution until boiling is initiated; continue with 10 min at a sub-boiling temperature (95°-98°C). Cool slides on bench top for 30 min.
Staining
1. Wash sections in dH2O three times for 5 min each.
2. Incubate sections in 3% hydrogen peroxide for 10 min.
3. Wash sections in dH2O two times for 5 min each.
4. Wash sections in wash buffer for 5 min.
5. Block each section with 100–400 µl of blocking solution for 1 hr at room temperature.
6. Remove blocking solution and add 100–400 µl primary antibody diluent in to each section. Incubate overnight at 4°C.
7. Remove antibody solution and wash sections with wash buffer three times for 5 min each.
8. Cover section with 1–3 drops HRPas needed. Incubate in a humidified chamber for 30 min at room temperature.
9. Wash sections three times with wash buffer for 5 min each.
10. Add DAB Chromogen Concentrate to DAB Diluent and mix well before use.
11. Apply 100–400 µl DAB to each section and monitor closely. 1–10 min generally provides an acceptable staining intensity.
12. Immerse slides in dH2O.
13. If desired, counterstain sections with hematoxylin.
14. Wash sections in dH2O two times for 5 min each.
15. Dehydrate sections: Incubate sections in 95% ethanol two times for 10 sec each; Repeat in 100% ethanol, incubating sections two times for 10 sec each; Repeat in xylene, incubating sections two times for 10 sec each.
16. Mount sections with coverslips and mounting medium.
|
Description biologique
| Spécificité |
|---|
| Tox/Tox2 Antibody [H10P3] detects endogenous levels of total Tox/Tox2 protein. |
| Localisation subcellulaire |
|---|
| Nucleus |
| Uniprot ID |
|---|
| Q96NM4; O94900 |
| Clone |
|---|
| H10P3 |
| Synonyme |
|---|
| TOX high mobility group box family member 2; Granulosa cell HMG box protein 1 (GCX-1); TOX2; C20orf100; GCX1; Thymocyte selection-associated high mobility group box protein TOX; Thymus high mobility group box protein TOX; TOX; KIAA0808 |
| Contexte |
|---|
| Tox (Thymocyte selection-associated high mobility group box protein) and its paralog Tox2 are conserved members of the HMG-box DNA-binding transcription factor subfamily, each featuring a central ~80-residue HMG domain comprising three α-helices that recognize AT-rich minor grooves through hydrophobic intercalation and electrostatic contacts with conserved basic residues such as Arg and Lys49. This domain is flanked by an N-terminal intrinsically disordered region that enables phase separation, and a C-terminal transactivation domain containing phosphorylation sites (e.g., Ser278 by ERK) and LXXLL motifs that recruit coactivators like CBP/p300, allowing Tox/Tox2 to bend DNA by ~90° and facilitate chromatin remodeling. Tox and Tox2 act as pioneer factors in T cell fate decisions: during thymic positive selection, Tox upregulates CD4-lineage genes (ThPOK, Gata3) by relieving Runx3 repression through HMG-mediated chromatin opening and synergy with NFAT/calcineurin, while Tox2 partners with Bcl6 and Stat3 in T follicular helper (Tfh) cell differentiation via a feed-forward loop that increases accessibility at Bcl6 loci and promotes germinal center formation. Both Tox and Tox2 mediate T cell exhaustion under chronic stimulation by maintaining expression of Pdcd1, Tim3, and Nr4a through persistent NFAT signaling despite Blimp1 antagonism. The ToxΔN isoform, resulting from leaky ribosome scanning at internal Met40/44, displays distinct cell cycle regulation compared to full-length ToxFL. Normally restricted to developing thymocytes and mature T/NK cells, Tox/Tox2 overexpression drives cutaneous T cell lymphoma and supports chronic infection persistence, while Tox deficiency impairs CD4, NKT, iNKT, LTi, and Treg development. |
| Références |
|---|
|
Support technique
Tel: +1-832-582-8158 Ext:3
Si vous avez dautres questions, veuillez laisser un message.