pour la recherche uniquement
GS-441524 Antiviral inhibiteur
Réf. CatalogueS6814
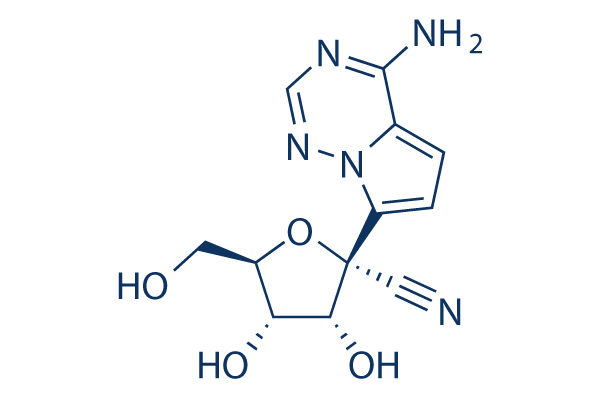
Structure chimique
Poids moléculaire: 291.26
Contrôle Qualité
| Cibles apparentées | Integrase Bacterial Antibiotics Anti-infection Fungal COVID-19 Parasite Reverse Transcriptase HIV HCV Protease |
|---|---|
| Autre Antiviral Inhibiteurs | Moroxydine HCl Aloperine Oleanolic Acid Harringtonine NGI-1 U18666A Aloin B Saikosaponin B2 Lapachol DTNB |
Culture cellulaire, traitement et concentration de travail
| Lignées cellulaires | Type dessai | Concentration | Temps dincubation | Formulation | Description de lactivité | PMID |
|---|---|---|---|---|---|---|
| HuH7 | Function assay | 10 uM | 24 hrs | Cmax in human HuH7 cells assessed as triphosphate concentration per million cells at 10 uM incubated for 24 hrs measured after washout by LC-MS analysis, Cmax=0.0000176μM | 22446091 | |
| Vero | Antiviral assay | 24 hrs | Antiviral activity against Parainfluenza 3 C 243 infected in african green monkey Vero cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=1.71μM | 22446091 | ||
| Vero | Antiviral assay | 24 hrs | Antiviral activity against SARS coronavirus Toronto-2 infected in african green monkey Vero cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=2.24μM | 22446091 | ||
| HuH7 | Antiviral assay | 24 hrs | Antiviral activity against Hepatitis C virus genotype 1b infected in human HuH7 cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=4.1μM | 22446091 | ||
| Vero E6 | Antiviral assay | 24 hrs | Antiviral activity against Dengue virus type 2 New Guinea C infected in african green monkey Vero E6 cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=9.46μM | 22446091 | ||
| HeLa | Antiviral assay | 24 hrs | Antiviral activity against Yellow fever virus 17D infected in human HeLa cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=11μM | 22446091 | ||
| MDCK | Antiviral assay | 24 hrs | Antiviral activity against Influenza A virus H3N2 infected in MDCK cells assessed as inhibition of virus induced cytopathic effect after 24 hrs by MTS assay, EC50=27.9μM | 22446091 | ||
| Hep2 | Antiviral assay | 4 days | Antiviral activity against Respiratory syncytial virus A2 infected in human Hep2 cells assessed as reduction of virus-induced cytopathic effect after 4 days by Cell-Titer Glo assay, EC50=0.53μM | 28124907 | ||
| HMVEC | Antiviral assay | 3 to 4 days | Antiviral activity against GFP-fused Ebolavirus infected in TERT-immortalized HMVEC assessed as reduction in viral replication preincubated with cells followed by viral infection measured after 3 to 4 days by fluorescence assay, EC50=0.78μM | 28124907 | ||
| HuH7 | Antiviral assay | 3 days | Antiviral activity against Hepatitis C virus genotype 1b infected in human HuH7 cells preincubated with cells for 3 days followed by viral infection by luciferase assay, EC50=4.1μM | 28124907 | ||
| HuH7 | Antiviral assay | 3 days | Antiviral activity against Ebolavirus infected in human HuH7 cells after 3 days by end point dilution assay, EC50=1.5μM | 28792763 | ||
| Cliquez pour voir plus de données expérimentales sur la lignée cellulaire | ||||||
Informations chimiques, stockage et stabilité
| Poids moléculaire | 291.26 | Formule | C12H13N5O4 |
Stockage (À compter de la date de réception) | 3 years -20°C powder |
|---|---|---|---|---|---|
| N° CAS | 1191237-69-0 | -- | Stockage des solutions mères |
|
|
| Synonymes | N/A | Smiles | C1=C2C(=NC=NN2C(=C1)C3(C(C(C(O3)CO)O)O)C#N)N | ||
Solubilité
|
In vitro |
DMSO
: 58 mg/mL
(199.13 mM)
Water : Insoluble Ethanol : Insoluble |
Calculateur de molarité
|
In vivo |
|||||
Calculateur de formulation in vivo (Solution claire)
Étape 1 : Saisir les informations ci-dessous (Recommandé : Un animal supplémentaire pour tenir compte des pertes pendant lexpérience)
Étape 2 : Saisir la formulation in vivo (Ceci est seulement le calculateur, pas la formulation. Veuillez nous contacter dabord sil ny a pas de formulation in vivo dans la section Solubilité.)
Résultats du calcul :
Concentration de travail : mg/ml;
Méthode de préparation du liquide maître DMSO : mg médicament prédissous dans μL DMSO ( Concentration du liquide maître mg/mL, Veuillez nous contacter dabord si la concentration dépasse la solubilité du DMSO du lot de médicament. )
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouterμL PEG300, mélanger et clarifier, puis ajouterμL Tween 80, mélanger et clarifier, puis ajouter μL ddH2O, mélanger et clarifier.
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouter μL Huile de maïs, mélanger et clarifier.
Note : 1. Veuillez vous assurer que le liquide est clair avant dajouter le solvant suivant.
2. Assurez-vous dajouter le(s) solvant(s) dans lordre. Vous devez vous assurer que la solution obtenue, lors de lajout précédent, est une solution claire avant de procéder à lajout du solvant suivant. Des méthodes physiques telles que le vortex, les ultrasons ou le bain-marie chaud peuvent être utilisées pour faciliter la dissolution.
Mécanisme daction
| Targets/IC50/Ki |
FIPV
(Cell-free assay) 0.78 μM(EC50)
|
Références |
|---|
Informations sur lessai clinique
(données du https://clinicaltrials.gov, mis à jour le 2024-05-22)
| Numéro NCT | Recrutement | Conditions | Promoteur/Collaborateurs | Date de début | Phases |
|---|---|---|---|---|---|
| NCT05996744 | Terminated | COVID-19 |
Gilead Sciences |
December 26 2023 | Phase 2|Phase 3 |
| NCT05715528 | Completed | COVID-19 |
Gilead Sciences |
February 8 2023 | Phase 3 |
| NCT05603143 | Terminated | COVID-19 |
Gilead Sciences |
November 5 2022 | Phase 3 |
| NCT04582266 | Completed | COVID-19 |
National Institute of Allergy and Infectious Diseases (NIAID)|Gilead Sciences |
March 31 2021 | -- |
| NCT04859244 | Completed | COVID-19 |
Copycat Sciences LLC |
January 1 2021 | Phase 1 |
| NCT04539262 | Completed | COVID-19 |
Gilead Sciences |
September 14 2020 | Phase 1|Phase 2 |
Support technique
Tel: +1-832-582-8158 Ext:3
Si vous avez dautres questions, veuillez laisser un message.
