pour la recherche uniquement
Aspirin COX inhibiteur
Réf. CatalogueS3017
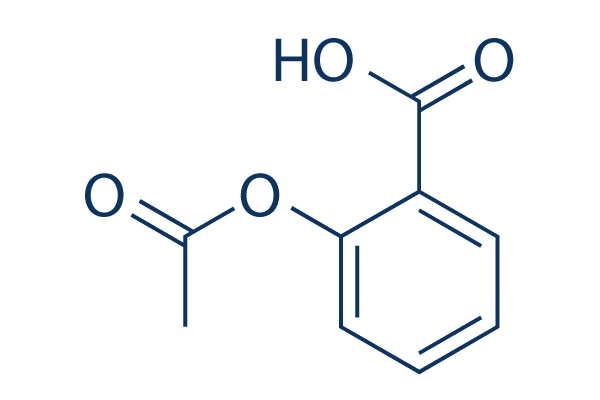
Structure chimique
Poids moléculaire: 180.16
Contrôle Qualité
Culture cellulaire, traitement et concentration de travail
| Lignées cellulaires | Type dessai | Concentration | Temps dincubation | Formulation | Description de lactivité | PMID |
|---|---|---|---|---|---|---|
| AGS | Function assay | 15 to 60 umol/L after | 12 hrs | Inhibition of Escherichia coli-stimulated IL-8 production in human AGS cells at 15 to 60 umol/L after 12 hrs by ELISA | 20153183 | |
| microglia cells | Antineuroinflammatory assay | 15 mins | Antineuroinflammatory activity in LPS-stimulated rat microglia cells assessed as inhibition of PMA-stimulated TXB2 release preincubated for 15 mins measured 70 mins after PMA challenge, IC50=3.12μM | 22153874 | ||
| HCT116 | Function assay | 1 mM | 6 hrs | Inhibition of TNF-alpha-induced NF-kappaB activation in human HCT116 cells at 1 mM after 6 hrs by luciferase reporter gene assay | 22154834 | |
| SKBR3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human SKBR3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| PANC1 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PANC1 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| PC3 | Growth inhibition assay | 300 uM | 48 hrs | Growth inhibition of human PC3 cells at 300 uM after 48 hrs by alamar blue assay | 22494617 | |
| MDA-MB-231 | Function assay | 100 uM | 30 mins | Irreversible inhibition of COX-1 in human MDA-MB-231 cells assessed as inhibition of arachidonic acid-induced PGE2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | 23651359 | |
| THP1 | Function assay | 100 uM | 30 mins | Irreversible inhibition of COX-1 in human THP1 cells assessed as inhibition of arachidonic acid-induced TXB2 formation at 100 uM incubated for 30 mins followed by compound washout measured 30 mins post arachidonic acid challenge by radioimmunoassay | 23651359 | |
| HCT116 | Cytotoxicity assay | 48 hrs | Cytotoxicity against human HCT116 cells assessed as reduction in cell viability after 48 hrs MTT assay | 26750401 | ||
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-6 in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of TNF-alpha in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by E | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring reduction in MDA levels at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in SOD activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in catalase activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of TNF-alpha in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-1beta in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as reduction in MDA levels at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by spectrophotometry | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress in rat H9c2 cells assessed as antioxidant activity by measuring increase in SOD activity at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by spec | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-6 in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by ELISA | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced oxidative stress mediated inflammation in rat H9c2 cells assessed as suppression of IL-1beta in supernatant at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs in presence of leonurine by EL | 27575471 | |
| H9c2 | Cardioprotective assay | 1 uM | 8 hrs | Cardioprotective activity against hypoxia-induced cytotoxicity in rat H9c2 cells assessed as increase in cell viability at 1 uM preincubated for 8 hrs followed by H2O2 addition for 2 hrs by MTT assay | 27575471 | |
| RAW264.7 | Antiinflammatory assay | Antiinflammatory activity in mouse RAW264.7 cells assessed as inhibition of LPS-induced nitric oxide production by measuring nitrite accumulation by Griess method, IC50=43.2μM | 28561586 | |||
| stem cells | Function assay | 100 uM | 5 days | Induction of adipogenesis in human bone marrow-derived mesenchymal stem cells assessed as increase in adiponectin production at 100 uM measured on day 5 in presence of IDX by ELISA | 29398443 | |
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| peritoneal cells | Function assay | 5 hrs | Inhibition of LPS-induced PGE2 production in C57BL6 mouse peritoneal cells measured at 5 hrs time interval by ELISA, IC50=4.08μM | 30006172 | ||
| HCT8 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human HCT8 cells after 72 hrs in presence of cinnamaldehyde by MTT assay, IC50=15.6μM | 30037494 | ||
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at G0/G1 phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as accumulation at S phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| RPMI8226 | Cell cycle assay | 1.7 uM | 48 hrs | Cell cycle arrest in human RPMI8226 cells assessed as reduction in accumulation at G2/M phase at 1.7 uM in presence of bortezomib after 48 hrs by propidium iodide/RNase staining based flow cytometric method relative to bortezomib | 30590258 | |
| HaCaT | Antipyretic assay | 100 uM | 2 hrs | Antipyretic activity in human HaCaT cells assessed as inhibition of TNFalpha-induced PGE2 production at 100 uM pre-incubated for 2 hrs before TNFalpha stimulation for 24 hrs by ELISA | 31393125 | |
| OVCAR5 | Function assay | 300 uM to 1 mM | 72 hrs | Inhibition of NAPRT in human OVCAR5 cells assessed as potentiation of NAMPT inhibitor FK866-induced cytotoxicity by measuring reduction in cell viability at 300 uM to 1 mM incubated for 72 hrs by SRB assay | ChEMBL | |
| CCRF-CEM | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human CCRF-CEM cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| Jurkat | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Jurkat cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further i | ChEMBL | |
| ML2 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human ML2 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further incu | ChEMBL | |
| NOMO | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NOMO cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| NB4 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NB4 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further incu | ChEMBL | |
| NAMALWA | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human NAMALWA cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| Daudi | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Daudi cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further in | ChEMBL | |
| Raji | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human Raji cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| ARH77 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human ARH77 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further in | ChEMBL | |
| RPMI8226 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human RPMI8226 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further | ChEMBL | |
| U266 | Function assay | 3.5 mM | 48 hrs | Inhibition of NAPRT in human U266 cells assessed as potentiation of NAMPT inhibitor FK866-induced apoptosis by measuring reduction in cell viability at 3.5 mM prer-incubated with IC50 level of FK866 for 48 hrs followed by compound addition and further inc | ChEMBL | |
| NAMALWA | Function assay | 35 mg/kg | 45 days | Potentiation of NAMPT inhibitor 10 mg/kg, ip bid FK866-induced antitumor activity in human NAMALWA cells xenografted in SCID mouse assessed as increase in mouse survival at 35 mg/kg, ip bid up to 45 days | ChEMBL | |
| Cliquez pour voir plus de données expérimentales sur la lignée cellulaire | ||||||
Informations chimiques, stockage et stabilité
| Poids moléculaire | 180.16 | Formule | C9H8O4 |
Stockage (À compter de la date de réception) | |
|---|---|---|---|---|---|
| N° CAS | 50-78-2 | Télécharger le SDF | Stockage des solutions mères |
|
|
| Synonymes | NSC 27223, Acetylsalicylic acid, ASA | Smiles | CC(=O)OC1=CC=CC=C1C(=O)O | ||
Solubilité
|
In vitro |
DMSO
: 36 mg/mL
(199.82 mM)
Ethanol : 36 mg/mL Water : Insoluble |
Calculateur de molarité
|
In vivo |
|||||
Calculateur de formulation in vivo (Solution claire)
Étape 1 : Saisir les informations ci-dessous (Recommandé : Un animal supplémentaire pour tenir compte des pertes pendant lexpérience)
Étape 2 : Saisir la formulation in vivo (Ceci est seulement le calculateur, pas la formulation. Veuillez nous contacter dabord sil ny a pas de formulation in vivo dans la section Solubilité.)
Résultats du calcul :
Concentration de travail : mg/ml;
Méthode de préparation du liquide maître DMSO : mg médicament prédissous dans μL DMSO ( Concentration du liquide maître mg/mL, Veuillez nous contacter dabord si la concentration dépasse la solubilité du DMSO du lot de médicament. )
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouterμL PEG300, mélanger et clarifier, puis ajouterμL Tween 80, mélanger et clarifier, puis ajouter μL ddH2O, mélanger et clarifier.
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouter μL Huile de maïs, mélanger et clarifier.
Note : 1. Veuillez vous assurer que le liquide est clair avant dajouter le solvant suivant.
2. Assurez-vous dajouter le(s) solvant(s) dans lordre. Vous devez vous assurer que la solution obtenue, lors de lajout précédent, est une solution claire avant de procéder à lajout du solvant suivant. Des méthodes physiques telles que le vortex, les ultrasons ou le bain-marie chaud peuvent être utilisées pour faciliter la dissolution.
Mécanisme daction
| Targets/IC50/Ki |
COX2
COX1
|
|---|---|
| In vitro |
L'Aspirin inhibe l'activation de NF-kappa B, empêchant ainsi la dégradation de l'inhibiteur de NF-kappa B, I kappa B, et par conséquent NF-kappa B est retenu dans le cytosol. Ce composé inhibe également la transcription dépendante de NF-kappa B de l'enhancer Ig kappa et de la répétition terminale longue (LTR) du virus de l'immunodéficience humaine (VIH) dans les cellules T transfectées. Son action et celle du salicylate sont médiatisées en partie par leur inhibition spécifique de l'IKK-bêta, empêchant ainsi l'activation par NF-kappaB des gènes impliqués dans la pathogenèse de la réponse inflammatoire. Ce produit chimique est protecteur contre la neurotoxicité provoquée par l'acide aminé excitateur glutamate dans les cultures neuronales primaires de rat et les tranches hippocampe. Il déclenche la biosynthèse transcellulaire d'une classe d'eicosanoïdes jusqu'alors inconnue lors de co-incubation de cellules endothéliales de la veine ombilicale humaine (HUVEC) et de neutrophiles [leucocytes polymorphonucléaires (PMN)]. Cet agent évoque une classe unique d'eicosanoïdes formés par des interactions entre PGHS-2 acétylée et 5-lipoxygénase. Son traitement inhibe la phosphorylation d'IRS-1 en Ser307 ainsi que la phosphorylation de JNK, c-Jun et la dégradation d'IkappaBalpha dans les cellules 3T3-L1 et Hep G2 traitées avec le facteur de nécrose tumorale (TNF)-alpha. Ce traitement inhibe la phosphorylation d'Akt et de la cible de la rapamycine chez les mammifères (mais pas de la kinase régulée par le signal extracellulaire ni de la PKCzeta) en réponse au TNF-alpha. Il restaure l'absorption de glucose induite par l'insuline dans les adipocytes 3T3-L1 prétraités au TNF-alpha.
|
Références |
|
Applications
| Méthodes | Biomarqueurs | Images | PMID |
|---|---|---|---|
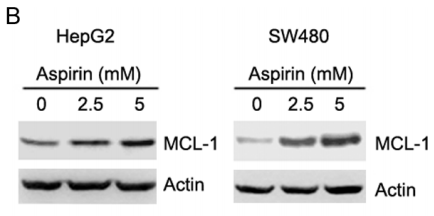
| Western blot | MCL-1 p-AKT / AKT / p-ERK / ERK p-AMPKα / AMPKα / p-ACC / ACC SHH / SMO / GLI1 / Bcl-2 / Foxm1 |
|
26918349 |
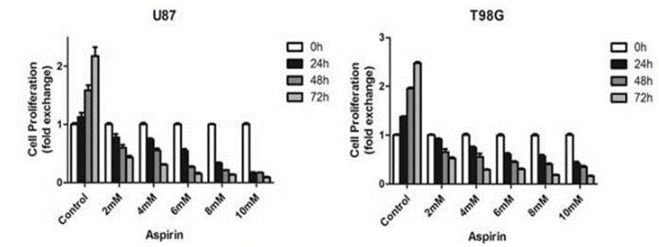
| Growth inhibition assay | Cell proliferation Cell viability |
|
28446712 |
Informations sur lessai clinique
(données du https://clinicaltrials.gov, mis à jour le 2024-05-22)
| Numéro NCT | Recrutement | Conditions | Promoteur/Collaborateurs | Date de début | Phases |
|---|---|---|---|---|---|
| NCT05960864 | Not yet recruiting | Ankylosing Spondylitis (AS) / Radiographic Axial SpA (r-axSpA)|Non-radiographic Axial Spondyloarthritis (Nr-axSpA)|Axial Psoriatic Arthritis (axPsA)|Acute Anterior Uveitis (AAU)|Crohn Disease (CD)|Ulcerative Colitis (UC)|Reactive Arthritis (ReA) |
Southwest Hospital China |
September 2024 | -- |
| NCT05868525 | Recruiting | Blunt Cerebrovascular Injury |
Loma Linda University |
July 2024 | Phase 4 |
| NCT06197009 | Not yet recruiting | Axial Spondyloarthritis |
Sinocelltech Ltd. |
July 1 2024 | Phase 2 |
| NCT06378398 | Not yet recruiting | Colorectal Neoplasia |
University of Michigan Rogel Cancer Center|National Cancer Institute (NCI) |
May 2024 | Early Phase 1 |
Support technique
Tel: +1-832-582-8158 Ext:3
Si vous avez dautres questions, veuillez laisser un message.
