pour la recherche uniquement
Dovitinib (TKI-258) FLT3 inhibiteur
Réf. CatalogueS1018
Structure chimique
Poids moléculaire: 392.43
Contrôle Qualité
| Cibles apparentées | EGFR VEGFR JAK PDGFR FGFR Src HIF FLT HER2 Bcr-Abl |
|---|---|
| Autre FLT3 Inhibiteurs | UNC2025 Crenolanib (CP-868596) Dovitinib (TKI258) Lactate monohydrate Tandutinib (MLN518) KW-2449 ENMD-2076 AST-487 (NVP-AST487) TCS 359 G-749 AMG 925 |
Culture cellulaire, traitement et concentration de travail
| Lignées cellulaires | Type dessai | Concentration | Temps dincubation | Formulation | Description de lactivité | PMID |
|---|---|---|---|---|---|---|
| SupB15 | Growth Inhibition Assay | IC50=0.449 μM | 25202073 | |||
| SupB15-R | Growth Inhibition Assay | IC50=0.558 μM | 25202073 | |||
| BaF3-pSRα | Growth Inhibition Assay | IC50=0.668 μM | 25202073 | |||
| BaF3-p210Bcr-Abl | Growth Inhibition Assay | IC50=0.692 μM | 25202073 | |||
| BaF3-p210Bcr-Abl-T315I | Growth Inhibition Assay | IC50=2.626 μM | 25202073 | |||
| CCRF-CEM | Growth Inhibition Assay | IC50=0.398 μM | 25202072 | |||
| CEM/C2 | Growth Inhibition Assay | IC50=1.125 μM | 25202072 | |||
| Nalm-6 | Growth Inhibition Assay | IC50=0.382 μM | 25202072 | |||
| SEM-K2 | Growth Inhibition Assay | IC50=0.022 μM | 25202072 | |||
| HB-1119 | Growth Inhibition Assay | IC50=0.028 μM | 25202072 | |||
| RS4:11 | Growth Inhibition Assay | IC50=2.81 μM | 25202072 | |||
| Nalm-6 | Apoptosis Assay | 2 μM | 24/48 h | induces apoptosis resulting in about 72% of cell death after 24 h treatment and 81% after 48 h | 25202072 | |
| SEM-K2 | Apoptosis Assay | 0.1/1 μM | 24 h | induces early apoptosis of SEM-K2 cells at 0.1 μM after 24 h | 25202072 | |
| HCT-116 | Growth Inhibition Assay | IC50=3.050.58 μM | 24495750 | |||
| HT-29 | Growth Inhibition Assay | IC50=5.21.93 μM | 24495750 | |||
| SW-480 | Growth Inhibition Assay | IC50=4.330.47 μM | 24495750 | |||
| CaCO2 | Growth Inhibition Assay | IC50=3.230.64 μM | 24495750 | |||
| LS174T | Growth Inhibition Assay | IC50=4.330.47 μM | 24495750 | |||
| HEC-1A | Function Assay | 0.05/0.1/0.5 μM | 72 h | causes a decrease in STAT3, ERK, and AKT phosphorylation | 24495750 | |
| AN3CA | Function Assay | 0.05/0.1/0.5 μM | 72 h | causes a decrease in STAT3, ERK, and AKT phosphorylation | 24495750 | |
| MFE-296 | Function Assay | 0.05/0.1/0.5 μM | 72 h | causes a decrease in STAT3, ERK, and AKT phosphorylation | 24495750 | |
| UMC3 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| 5637 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| HU456 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| MGHU4 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| HT1376 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| RT112 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| T24 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| BFTC905 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| TCC-SUP | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| RT4 | Cell Viability Assay | 1-10 μM | 72 h | inhibits cell growth in a dose dependent manner | 24325461 | |
| HONE1 | Growth Inhibition Assay | 0.1-10 μM | 48 h | induces G2/M delay in a concentration-dependent manner | 24238094 | |
| HNE1 | Growth Inhibition Assay | 0.1-10 μM | 48 h | induces G2/M delay in a concentration-dependent manner | 24238094 | |
| CNE2 | Growth Inhibition Assay | 0.1-10 μM | 48 h | induces G2/M delay in a concentration-dependent manner | 24238094 | |
| C666-1 | Growth Inhibition Assay | 0.1-10 μM | 48 h | induces G2/M delay in a concentration-dependent manner | 24238094 | |
| HeLa | Growth Inhibition Assay | 0.1-10 μM | 24 h | induces G2/M arrest in a concentration-dependent manner | 24238094 | |
| Hep3B | Growth Inhibition Assay | 0.1-10 μM | 24 h | induces G2 arrest | 24238094 | |
| HepG2 | Growth Inhibition Assay | 48 h | IC50=2.727 ± 0.429 μM | 23546591 | ||
| Hep3B | Growth Inhibition Assay | 48 h | IC50=4.223 ± 0.839 μM | 23546591 | ||
| PLC/PRF5 | Growth Inhibition Assay | 48 h | IC50=16.120 ± 4.001 μM | 23546591 | ||
| Huh7 | Growth Inhibition Assay | 48 h | IC50=15.007 ± 7.334 μM | 23546591 | ||
| HepG2 | Growth Inhibition Assay | 72 h | IC50=1.200 ± 0.226 μM | 23546591 | ||
| Hep3B | Growth Inhibition Assay | 72 h | IC50=0.892 ± 0.044 μM | 23546591 | ||
| PLC/PRF5 | Growth Inhibition Assay | 72 h | IC50=3.110 ± 0.337 μM | 23546591 | ||
| Huh7 | Growth Inhibition Assay | 72 h | IC50=3.980 ± 0.803 μM | 23546591 | ||
| MFE280 | Growth Inhibition Assay | IC50=0.42 ± 0.06 μM | 23443805 | |||
| AN3CA | Growth Inhibition Assay | IC50=0.50 ± 0.10 μM | 23443805 | |||
| HEC155 | Growth Inhibition Assay | IC50=0.66 ± 0.09 μM | 23443805 | |||
| MFE296 | Growth Inhibition Assay | IC50=0.66 ± 0.19 μM | 23443805 | |||
| SPAC1S | Growth Inhibition Assay | IC50=0.77 ± 0.08 μM | 23443805 | |||
| RL952 | Growth Inhibition Assay | IC50=0.93 ± 0.01 μM | 23443805 | |||
| EN1 | Growth Inhibition Assay | IC50=1.02 ± 0.25 μM | 23443805 | |||
| SNGII | Growth Inhibition Assay | IC50=1.24 ± 0.28 μM | 23443805 | |||
| ISHIKAWA | Growth Inhibition Assay | IC50=1.30 ± 0.11 μM | 23443805 | |||
| HEC1A | Growth Inhibition Assay | IC50=1.34 ± 0.30 μM | 23443805 | |||
| KLE | Growth Inhibition Assay | IC50=1.37 ± 0.02 μM | 23443805 | |||
| SNGM | Growth Inhibition Assay | IC50=1.42 ± 0.13 μM | 23443805 | |||
| USPC2 | Growth Inhibition Assay | IC50=1.62 ± 0.01 μM | 23443805 | |||
| EN | Growth Inhibition Assay | IC50=1.66 ± 0.01 μM | 23443805 | |||
| MFE319 | Growth Inhibition Assay | IC50=1.87 ± 0.45 μM | 23443805 | |||
| EFE184 | Growth Inhibition Assay | IC50=2.04 ± 0.13 μM | 23443805 | |||
| ECC1 | Growth Inhibition Assay | IC50=2.07 ± 0.01 μM | 23443805 | |||
| HEC1B | Growth Inhibition Assay | IC50=2.57 ± 0.23 μM | 23443805 | |||
| USPC1 | Growth Inhibition Assay | IC50=2.60 ± 0.13 μM | 23443805 | |||
| SPAC1L | Growth Inhibition Assay | IC50=3.06 ± 1.14 μM | 23443805 | |||
| HUVEC | Cell Viability Assay | 0-25 μM | 72 h | DMSO | inhibits cell growth in a dose dependent manner | 23228017 |
| HMVEC | Cell Viability Assay | 0-25 μM | 72 h | DMSO | inhibits cell growth in a dose dependent manner | 23228017 |
| MHCC-97H | Cell Viability Assay | 0-25 μM | 72 h | DMSO | inhibits cell growth in a dose dependent manner | 23228017 |
| SMMC7721 | Cell Viability Assay | 0-25 μM | 72 h | DMSO | inhibits cell growth in a dose dependent manner | 23228017 |
| Huh-7 | Apoptosis Assay | 0-12.5 μM | 24 h | DMSO | sensitizes HCC cells to TRAIL- and tigatuzumab-induced apoptosis in a dose-dependent manner | 22230479 |
| Sk-Hep1 | Apoptosis Assay | 0-12.5 μM | 24 h | DMSO | sensitizes HCC cells to TRAIL- and tigatuzumab-induced apoptosis in a dose-dependent manner | 22230479 |
| Hep3B | Apoptosis Assay | 0-12.5 μM | 24 h | DMSO | sensitizes HCC cells to TRAIL- and tigatuzumab-induced apoptosis in a dose-dependent manner | 22230479 |
| PLC5 | Apoptosis Assay | 0-12.5 μM | 24 h | DMSO | sensitizes HCC cells to TRAIL- and tigatuzumab-induced apoptosis in a dose-dependent manner | 22230479 |
| PLC5 | Cell Viability Assay | 0-15 μM | 72 h | reduces cell viability in a dose-dependent manner | 22180308 | |
| Hep3B | Cell Viability Assay | 0-15 μM | 72 h | reduces cell viability in a dose-dependent manner | 22180308 | |
| Sk-Hep1 | Cell Viability Assay | 0-15 μM | 72 h | reduces cell viability in a dose-dependent manner | 22180308 | |
| Huh-7 | Cell Viability Assay | 0-15 μM | 72 h | reduces cell viability in a dose-dependent manner | 22180308 | |
| PLC5 | Apoptosis Assay | 0-15 μM | 24 h | increases apoptotic cell death in a dose-dependent manner | 22180308 | |
| Hep3B | Apoptosis Assay | 0-15 μM | 24 h | increases apoptotic cell death in a dose-dependent manner | 22180308 | |
| Sk-Hep1 | Apoptosis Assay | 0-15 μM | 24 h | increases apoptotic cell death in a dose-dependent manner | 22180308 | |
| Huh-7 | Apoptosis Assay | 0-15 μM | 24 h | increases apoptotic cell death in a dose-dependent manner | 22180308 | |
| PLC5 | Function Assay | 0-10 μM | 24 h | causes dose-dependent DNA fragmentation | 22180308 | |
| Hep3B | Function Assay | 0-10 μM | 24 h | causes dose-dependent DNA fragmentation | 22180308 | |
| Sk-Hep1 | Function Assay | 0-10 μM | 24 h | causes dose-dependent DNA fragmentation | 22180308 | |
| Huh-7 | Function Assay | 0-10 μM | 24 h | causes dose-dependent DNA fragmentation | 22180308 | |
| SW780 | Growth Inhibition Assay | 5 d | IC50=50 nM | 21119661 | ||
| RT112 | Growth Inhibition Assay | 5 d | IC50=15 nM | 21119661 | ||
| RT4 | Growth Inhibition Assay | 5 d | IC50=5 nM | 21119661 | ||
| JMSU1 | Growth Inhibition Assay | 5 d | IC50=50 nM | 21119661 | ||
| J82 | Growth Inhibition Assay | 5 d | IC50=1400 nM | 21119661 | ||
| 97-7 | Growth Inhibition Assay | 5 d | IC50=1000 nM | 21119661 | ||
| RT112 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| RT4 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| MGH-U3 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| SW780 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| 97-7 | Function Assay | 500 nM | 24 h | increases the proportion of cells in G1 accompanied by a decrease in S and G2/M phases | 21119661 | |
| J807C | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| Y373C | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| K650E | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| G384D | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| F384L | Cell Viability Assay | 0-400 nM | 48 h | inhibits cell growth in a dose dependent manner | 15598814 | |
| KMS11 | Growth Inhibition Assay | 72 h | IC50=90 nM | 15598814 | ||
| KMS18 | Growth Inhibition Assay | 72 h | IC50=550 nM | 15598814 | ||
| OPM2 | Growth Inhibition Assay | 72 h | IC50=90 nM | 15598814 | ||
| H929 | Growth Inhibition Assay | 72 h | IC50> 2500 nM | 15598814 | ||
| 8226 | Growth Inhibition Assay | 72 h | IC50> 2500 nM | 15598814 | ||
| U266 | Growth Inhibition Assay | 72 h | IC50> 2500 nM | 15598814 | ||
| KM12L4A | Function assay | Inhibition of VEGFR2 phosphorylation expressed in human KM12L4A cells by Western blot analysis, EC50=0.046μM | 19113866 | |||
| KM12L4A | Function assay | Inhibition of PDGFRbeta phosphorylation expressed in human KM12L4A cells Western blot analysis, EC50=0.051μM | 19113866 | |||
| KM12L4A | Function assay | Inhibition of FGFR1 phosphorylation expressed in human KM12L4A cells by Western blot analysis, EC50=0.166μM | 19113866 | |||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant PDGFRbeta (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGLFDDPSYVNVQNL in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.001μM | 27914362 | ||
| Sf9 | Function assay | 1 to 4 hrs | Inhibition of recombinant human N-terminal GST/His6-tagged c-KIT (544 to 976 residues) expressed in baculovirus infected sf9 cells using biotinylated peptide substrate GGLFDDPSYVNVQNL in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescen, IC50=0.001μM | 27914362 | ||
| Sf9 | Function assay | 1 to 4 hrs | Inhibition of recombinant human N-terminal GST/His6-tagged FLT3 (571 to 993 residues) expressed in baculovirus infected sf9 cells using biotinylated peptide substrate GGLFDDPSYVNVQNL in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescenc, IC50=0.001μM | 27914362 | ||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant FGFR1 (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGGGQDGKDYIVLPI in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.008μM | 27914362 | ||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant VEGFR3 (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGGGQDGKDYIVLPI in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.008μM | 27914362 | ||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant VEGFR1 (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGGGQDGKDYIVLPI in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.01μM | 27914362 | ||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant VEGFR2 (unknown origin) expressed in baculovirus infected insect cells using biotinylated peptide substrate GGGGQDGKDYIVLPI in presence of ATP incubated for 1 to 4 hrs by time resolved fluorescence assay, IC50=0.013μM | 27914362 | ||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| insect cells | Function assay | 1 to 4 hrs | Inhibition of recombinant FGFR1 (unknown origin) expressed in baculovirus infected insect cells using GGGGQDGKDYIVLPI as substrate after 1 to 4 hrs by time-resolved fluorescence assay, IC50=0.008μM | 30503938 | ||
| NCI-H1703 | Function assay | 10 uM | 24 hrs | Inhibition of TNIK in human NCI-H1703 cells transfected with lentiviral vector 7TFP assessed as reduction of GSK3 inhibitor X activated TNIK-mediated Wnt/TCF/beta-catenin-dependent transcription at 10 uM after 24 hrs by luciferase reporter assay | ChEMBL | |
| LoVo | Cytotoxicity assay | 10 uM | 72 hrs | Cytotoxicity against Wnt/beta-catenin signalling dependent human LoVo cells assessed as cell viability at 10 uM after 72 hrs by ATPlite assay | ChEMBL | |
| HCT116 | Cytotoxicity assay | 10 uM | 72 hrs | Cytotoxicity against Wnt/beta-catenin signalling dependent human HCT116 cells assessed as cell viability at 10 uM after 72 hrs by ATPlite assay | ChEMBL | |
| Cliquez pour voir plus de données expérimentales sur la lignée cellulaire | ||||||
Informations chimiques, stockage et stabilité
| Poids moléculaire | 392.43 | Formule | C21H21FN6O |
Stockage (À compter de la date de réception) | |
|---|---|---|---|---|---|
| N° CAS | 405169-16-6 | Télécharger le SDF | Stockage des solutions mères |
|
|
| Synonymes | CHIR-258 | Smiles | CN1CCN(CC1)C2=CC3=C(C=C2)N=C(N3)C4=C(C5=C(C=CC=C5F)NC4=O)N | ||
Solubilité
|
In vitro |
DMSO
: 30 mg/mL
(76.44 mM)
Water : Insoluble Ethanol : Insoluble |
Calculateur de molarité
|
In vivo |
|||||
Calculateur de formulation in vivo (Solution claire)
Étape 1 : Saisir les informations ci-dessous (Recommandé : Un animal supplémentaire pour tenir compte des pertes pendant lexpérience)
Étape 2 : Saisir la formulation in vivo (Ceci est seulement le calculateur, pas la formulation. Veuillez nous contacter dabord sil ny a pas de formulation in vivo dans la section Solubilité.)
Résultats du calcul :
Concentration de travail : mg/ml;
Méthode de préparation du liquide maître DMSO : mg médicament prédissous dans μL DMSO ( Concentration du liquide maître mg/mL, Veuillez nous contacter dabord si la concentration dépasse la solubilité du DMSO du lot de médicament. )
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouterμL PEG300, mélanger et clarifier, puis ajouterμL Tween 80, mélanger et clarifier, puis ajouter μL ddH2O, mélanger et clarifier.
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouter μL Huile de maïs, mélanger et clarifier.
Note : 1. Veuillez vous assurer que le liquide est clair avant dajouter le solvant suivant.
2. Assurez-vous dajouter le(s) solvant(s) dans lordre. Vous devez vous assurer que la solution obtenue, lors de lajout précédent, est une solution claire avant de procéder à lajout du solvant suivant. Des méthodes physiques telles que le vortex, les ultrasons ou le bain-marie chaud peuvent être utilisées pour faciliter la dissolution.
Mécanisme daction
| Targets/IC50/Ki |
FLT3
(Cell-free assay) 1 nM
c-Kit
(Cell-free assay) 2 nM
FGFR1
(Cell-free assay) 8 nM
VEGFR3/FLT4
(Cell-free assay) 8 nM
FGFR3
(Cell-free assay) 9 nM
VEGFR1/FLT1
(Cell-free assay) 10 nM
VEGFR2/Flk1
(Cell-free assay) 13 nM
PDGFRβ
(Cell-free assay) 27 nM
CSF-1R/c-Fms
(Cell-free assay) 36 nM
|
|---|---|
| In vitro |
Dovitinib (TKI-258) inhibe puissamment la croissance stimulée par le FGF des cellules B9 exprimant WT et F384L-FGFR3 avec une IC50 de 25 nM. De plus, il inhibe la prolifération des cellules B9 exprimant chacune des diverses mutations activées de FGFR3. Fait intéressant, des différences minimales sont observées dans la sensibilité des différentes mutations de FGFR3 à ce composé, avec une IC50 allant de 70 à 90 nM pour chacune des diverses mutations. Les cellules B9 dépendantes de l'IL-6 contenant uniquement le vecteur (cellules B9-MINV) sont résistantes à son activité inhibitrice à des concentrations allant jusqu'à 1 µM. Il inhibe la prolifération cellulaire des cellules KMS11 (FGFR3-Y373C), OPM2 (FGFR3-K650E) et KMS18 (FGFR3-G384D) avec une IC50 de 90 nM (KMS11 et OPM2) et 550 nM, respectivement. Le composé inhibe la phosphorylation d'ERK1/2 médiée par le FGF et induit une cytotoxicité dans les cellules MM primaires exprimant FGFR3. Les BMSC confèrent un degré modeste de résistance avec une inhibition de croissance de 44,6 % pour les cellules traitées avec 500 nM de Dovitinib et cultivées sur stroma, comparativement à une inhibition de croissance de 71,6 % pour les cellules cultivées sans BMSC. Il inhibe la prolifération de M-NFS-60, une lignée cellulaire myéloblastique de souris dont la croissance est stimulée par le M-CSF, avec une concentration efficace médiane (EC50) de 220 nM. Le traitement des cellules SK-HEP1 avec ce composé entraîne une réduction dose-dépendante du nombre de cellules et un arrêt en phase G2/M avec une réduction des phases G0/G1 et S, une inhibition de la croissance indépendante de l'ancrage et un blocage de la motilité cellulaire induite par le bFGF. L'IC50 pour ce composé dans les cellules SK-HEP1 est d'environ 1,7 µM. Il réduit également significativement les niveaux de phosphorylation basaux de FGFR-1, du substrat 2α de FGFR (FRS2-α) et d'ERK1/2, mais pas d'Akt, dans les cellules SK-HEP1 et 21-0208. Dans les cellules HCC 21-0208, il inhibe significativement la phosphorylation de FGFR-1, FRS2-α, ERK1/2 induite par le bFGF, mais pas d'Akt. |
| Essai kinase |
Essais kinases in vitro
|
|
Les valeurs de concentration inhibitrice de 50 % (IC50) pour l'inhibition des RTK par Dovitinib (TKI-258) sont déterminées dans un format de fluorescence résolue dans le temps (TRF) ou radioactif, mesurant son inhibition du transfert de phosphate vers un substrat par l'enzyme respective. Les domaines kinase de FGFR3, FGFR1, PDGFRβ et VEGFR1-3 sont testés dans 50 mM HEPES (acide N-2-hydroxyéthylpipérazine-N′-2-éthanesulfonique), pH 7,0, 2 mM MgCl2, 10 mM MnCl2, 1 mM NaF, 1 mM dithiothréitol (DTT), 1 mg/mL d'albumine sérique bovine (BSA), 0,25 μM de substrat peptidique biotinylé (GGGGQDGKDYIVLPI) et 1 à 30 μM d'adénosine triphosphate (ATP) selon le Km de l'enzyme respective. Les concentrations d'ATP sont égales ou légèrement inférieures au Km. Pour les réactions c-KIT et FLT3, le pH est augmenté à 7,5 avec 0,2 à 8 μM d'ATP en présence de 0,25 à 1 μM de substrat peptidique biotinylé (GGLFDDPSYVNVQNL). Les réactions sont incubées à température ambiante pendant 1 à 4 heures et le peptide phosphorylé est capturé sur des plaques de microtitration recouvertes de streptavidine contenant un tampon d'arrêt de réaction (25 mM EDTA [acide éthylènediaminetétraacétique], 50 mM HEPES, pH 7,5). Le peptide phosphorylé est mesuré avec le système DELFIA TRF utilisant un anticorps anti-phosphotyrosine PT66 marqué à l'europium. La concentration de ce composé pour l'IC50 est calculée à l'aide d'une régression non linéaire avec le logiciel d'analyse de données XL-Fit version 4.1 (IDBS). L'inhibition de l'activité kinase du récepteur du facteur stimulant les colonies-1 (CSF-1R), PDGFRα, du récepteur de l'insuline (InsR) et du récepteur du facteur de croissance analogue à l'insuline 1 (IGFR1) est déterminée à des concentrations d'ATP proches du Km pour l'ATP.
|
|
| In vivo |
Dovitinib (TKI-258) induit des réponses à la fois cytostatiques et cytotoxiques in vivo, entraînant la régression des tumeurs exprimant FGFR3. Il montre une inhibition dose-dépendante et exposition-dépendante des récepteurs tyrosine kinases (RTK) cibles exprimés dans les xénogreffes tumorales. Ce composé inhibe puissamment la croissance tumorale de six lignées de CHC. L'inhibition de l'Angiogenesis est corrélée à l'inactivation des voies de signalisation FGFR/PDGFRβ/VEGFR2. Dans un modèle orthotopique, il inhibe puissamment la croissance tumorale primaire et les métastases pulmonaires et prolonge significativement la survie des souris. L'administration de Dovitinib entraîne une inhibition significative de la croissance tumorale et des régressions tumorales, y compris des tumeurs grandes et établies (500-1 000 mm3). |
Références |
|
Applications
| Méthodes | Biomarqueurs | Images | PMID |
|---|---|---|---|
| Western blot | CDK1 / p-CDK1 / p53 / p21 p-PDGFR-β / PDGFR-β / p-ERK / ERK p-VEGFR-2 / VEGFR-2 / p-FGFR-1 / FGFR-1 p-STAT3 / STAT3 / Mcl-1 / LC3 / Beclin 1 / p62 |
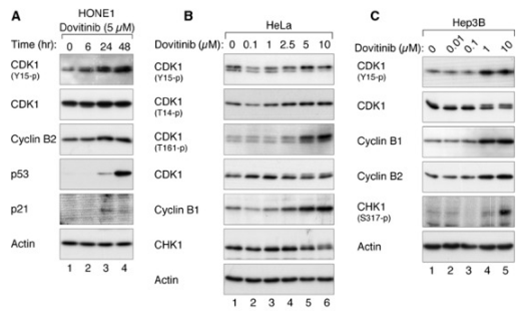
|
24238094 |
| Growth inhibition assay | Cell viability |
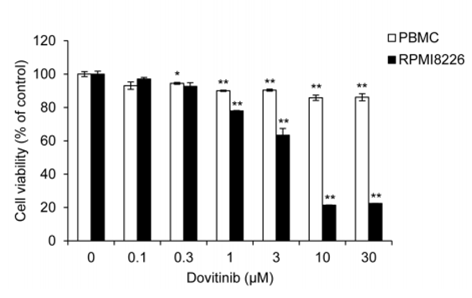
|
28467797 |
Informations sur lessai clinique
(données du https://clinicaltrials.gov, mis à jour le 2024-05-22)
| Numéro NCT | Recrutement | Conditions | Promoteur/Collaborateurs | Date de début | Phases |
|---|---|---|---|---|---|
| NCT05571969 | Recruiting | Advanced Solid Tumors |
Allarity Therapeutics|Amarex Clinical Research |
February 20 2023 | Phase 1 |
| NCT02268435 | Withdrawn | Gastrointestinal Stromal Tumors |
Asan Medical Center |
March 2015 | Phase 1 |
| NCT01700270 | Completed | Advanced Solid Tumors Excluding Breast Cancer |
Novartis Pharmaceuticals|Novartis |
May 2013 | Phase 1 |
| NCT01680796 | Withdrawn | Multiple Myeloma |
University of Florida|Novartis Pharmaceuticals |
February 2013 | Phase 1 |
| NCT01266070 | Terminated | Von Hippel-Lindau Syndrome |
M.D. Anderson Cancer Center|Novartis |
November 2012 | Phase 2 |
Support technique
Tel: +1-832-582-8158 Ext:3
Si vous avez dautres questions, veuillez laisser un message.
