pour la recherche uniquement
Decitabine DNA Methyltransferase inhibiteur
Réf. CatalogueS1200
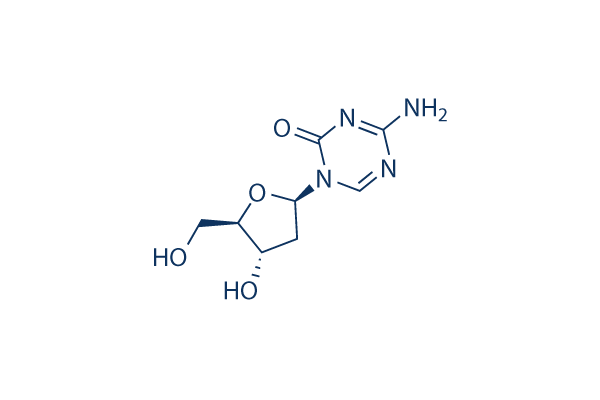
Structure chimique
Poids moléculaire: 228.21
Contrôle Qualité
| Cibles apparentées | HDAC JAK BET Histone Methyltransferase PKC PARP HIF PRMT EZH2 AMPK |
|---|---|
| Autre DNA Methyltransferase Inhibiteurs | RG108 SGI-1027 Zebularine (NSC 309132) GSK3685032 Gamma-Oryzanol CM272 β-thujaplicin Bobcat339 DC-05 2'-Deoxy-5-Fluorocytidine |
Culture cellulaire, traitement et concentration de travail
| Lignées cellulaires | Type dessai | Concentration | Temps dincubation | Formulation | Description de lactivité | PMID |
|---|---|---|---|---|---|---|
| Eca109 | Function Assay | 0.5 μM | 24 h | water | modulates the gene expression of MAGE-A members | 25123082 |
| Eca109 | Growth Inhibition Assay | 0.5/2.5/5 μM | 24/48/72 h | water | inhibits cell growth in both dose- and time- dependent manner | 25123082 |
| Eca109 | Function Assay | 0.5 μM | 6/12/24 h | water | inhibits cell migration | 25123082 |
| Eca109 | Function Assay | 0.5 μM | 24 h | water | inhibits cell invasion | 25123082 |
| Eca109 | Growth Inhibition Assay | 0.5 μM | 24 h | water | induces G2/M arrest in the cell cycle | 25123082 |
| Eca109 | Function Assay | 0.5/1 μM | 24 h | water | decreases expression of NF-κB2 and MMP2 | 25123082 |
| SW1116 | Growth Inhibition Assay | 0.5/1/2/5 μM | 48 h | DMSO | enhances the Gefitinib induced cell inhibition | 24874286 |
| LOVO | Growth Inhibition Assay | 0.5/1/2/5 μM | 48 h | DMSO | enhances the Gefitinib induced cell inhibition | 24874286 |
| SW1116 | Function Assay | 10 μM | 48 h | DMSO | increases the effective at inhibiting AKT and mTOR signaling pathways combined with gefitinib | 24874286 |
| LOVO | Function Assay | 10 μM | 48 h | DMSO | increases the effective at inhibiting AKT and mTOR signaling pathways combined with gefitinib | 24874286 |
| SW1116 | Apoptosis Assay | 10 μM | 48 h | DMSO | enhances Gefitinib-induced apoptosis | 24874286 |
| LOVO | Apoptosis Assay | 10 μM | 48 h | DMSO | enhances Gefitinib-induced apoptosis | 24874286 |
| RPMI-8226 | Apoptosis Assay | 1/2 μM | 48/72/96 h | DMSO | induces cell apoptosis | 24833108 |
| OPM-2 | Apoptosis Assay | 1/2 μM | 72/96/120 h | DMSO | induces cell apoptosis | 24833108 |
| JJN3 | Apoptosis Assay | 0.5/1 μM | 24/48 h | DMSO | induces cell apoptosis | 24833108 |
| NCI-H929 | Apoptosis Assay | 1/2 μM | 72/96/120 h | DMSO | induces cell apoptosis | 24833108 |
| RPMI-8226 | Growth Inhibition Assay | 1/2 μM | 24/48/72 h | DMSO | affects cell cycle progression negatively | 24833108 |
| OPM-2 | Growth Inhibition Assay | 1/2 μM | 24/48/72 h | DMSO | affects cell cycle progression negatively | 24833108 |
| JJN3 | Growth Inhibition Assay | 0.5/1 μM | 24/48/72 h | DMSO | affects cell cycle progression negatively | 24833108 |
| NCI-H929 | Growth Inhibition Assay | 1/2 μM | 24/48/72 h | DMSO | affects cell cycle progression negatively | 24833108 |
| HeLa | Kinase Assay | Ki=1000–5000 μM for hENT1 | 24780098 | |||
| HeLa | Kinase Assay | Ki=5.6 ± 0.5 μM for hENT2 | 24780098 | |||
| HeLa | Kinase Assay | Ki=21.6 ± 3.0 μM for hCNT1 | 24780098 | |||
| HeLa | Kinase Assay | Ki=14.4 ± 4.6 μM for hCNT3 | 24780098 | |||
| NB4 | Function Assay | 2.5/5/7.5/10 μM | 24 h | DMSO | increasea the expression of precursor miR-125a | 24484870 |
| CD4+ CD25− T | Function Assay | 1/5 μM | reduceS global DNA methylation | 24476360 | ||
| BV-173 | Apoptosis Assay | 0.25/0.5/0.75/1 μM | 48/72/96 h | PBS | induces cell apoptosis in both dose- and time- dependent manner | 24423613 |
| ML-1 | Apoptosis Assay | 0.25/0.5/0.75/1 μM | 48/72/96 h | PBS | induces cell apoptosis in both dose- and time- dependent manner | 24423613 |
| HL-60 | Apoptosis Assay | 0.25/0.5/0.75/1 μM | 48/72/96 h | PBS | induces cell apoptosis in both dose- and time- dependent manner | 24423613 |
| KG-1a | Apoptosis Assay | 0.25/0.5/0.75/1 μM | 48/72/96 h | PBS | induces cell apoptosis in both dose- and time- dependent manner | 24423613 |
| BV-173 | Function Assay | 250/500nM | 48 h | PBS | induces delayed and sustained ROS increase | 24423613 |
| CEM | Function Assay | 250/500nM | 48 h | PBS | induces delayed and sustained ROS increase | 24423613 |
| HL-60 | Function Assay | 250/500nM | 48 h | PBS | induces delayed and sustained ROS increase | 24423613 |
| ML-1 | Function Assay | 250/500nM | 48 h | PBS | induces delayed and sustained ROS increase | 24423613 |
| DLD-1 | Function Assay | 250/500nM | 48 h | PBS | do not induces delayed and sustained ROS increase | 24423613 |
| HCT-116 | Function Assay | 250/500nM | 48 h | PBS | do not induces delayed and sustained ROS increase | 24423613 |
| U937-A/E-9/14/18 | Apoptosis Assay | 0.01/0.1/1/10 μM | 48 h | induces cell apoptosis in a dose-dependent manner | 24300456 | |
| HT29 | Growth Inhibition Assay | 72 h | IC50=1400±179 μM | 24172061 | ||
| SW48 | Growth Inhibition Assay | 72 h | IC50=15.2±6.2 μM | 24172061 | ||
| HCT116 | Growth Inhibition Assay | 72 h | IC50=1.7±0.4 μM | 24172061 | ||
| HepG2 | Function Assay | 0.5/1 μM | 24 h | DMSO | up-regulated the relative OCTN2 mRNA and protein expression | 24146874 |
| LS174T | Function Assay | 0.5/1 μM | 24 h | DMSO | lead to an increase of OCTN2 levels | 24146874 |
| HepG2 | Apoptosis Assay | 1/10/100 μM | 7 d | DMSO | induces cell apoptosis in a dose-dependent manner | 24146874 |
| LS174T | Apoptosis Assay | 1/10/100 μM | 7 d | DMSO | induces cell apoptosis in a dose-dependent manner | 24146874 |
| QBC-939 | Apoptosis Assay | 1/10/100 μM | 7 d | DMSO | induces cell apoptosis in a dose-dependent manner | 24146874 |
| U251 | Apoptosis Assay | 1/10/100 μM | 7 d | DMSO | induces cell apoptosis in a dose-dependent manner | 24146874 |
| HL-60 | Growth Inhibition Assay | 1 μM | 48 h | increases G2-phase cell fraction | 24000324 | |
| MDA‑MB‑453 | Function Assay | 0.2/1 μM | 72 h | causes re-expression of claudin 1 | 23844228 | |
| HCC1569 | Function Assay | 0.2/1 μM | 72 h | causes re-expression of claudin 1 | 23844228 | |
| BT‑474 | Function Assay | 0.2/1 μM | 72 h | causes re-expression of claudin 1 | 23844228 | |
| AGS | Apoptosis Assay | 5/10/20/50 μM | 48 h | DMSO | inhibits the cell viability | 23582784 |
| A549 | Apoptosis Assay | 5/10/20/50 μM | 48 h | DMSO | inhibits the cell viability | 23582784 |
| AGS | Growth Inhibition Assay | 5/10/20/50 μM | 48 h | DMSO | induces G2/M phase arrest | 23582784 |
| Kasumi-1 | Apoptosis Assay | 0.5 μM | 48 h | decreases the cell viability co-treated with Tf-NP-sc | 23493348 | |
| OCI-AML3 | Apoptosis Assay | 2.5 μM | 48 h | decreases the cell viability co-treated with Tf-NP-sc | 23493348 | |
| MV4-11 | Apoptosis Assay | 2.5 μM | 48 h | decreases the cell viability co-treated with Tf-NP-sc | 23493348 | |
| NK | Cytotoxity Assay | 0.02-20 μM | 5 d | decreases the cytolytic activity of NK cells at intermediate concentrations resulting in a U-shaped dose–response curve | 23328088 | |
| NK | Apoptosis Assay | 0.02-20 μM | 5 d | decrease NK cell proliferation and viability as the concentration increased | 23328088 | |
| NK | Function Assay | 0.01-20 μM | 5 d | causes hypomethylation of NK cells in a dose–response | 23328088 | |
| MOLT4/DNR | Function Assay | 5 μM | 4 d | reduces ABCB1 mRNA expression | 23060570 | |
| Jurkat/DOX | Function Assay | 5 μM | 4 d | reduces ABCB1 mRNA expression | 23060570 | |
| MOLT4/DNR | Growth Inhibition Assay | 5 μM | 4 d | reduces the IC50 value for daunorubicin sensitivity | 23060570 | |
| Jurkat/DOX | Growth Inhibition Assay | 5 μM | 4 d | reduces the IC50 value for daunorubicin sensitivity | 23060570 | |
| ccRCC | Apoptosis Assay | 0.01-10μM | 72 h | DMSO | has minimal effect on cell proliferation | 22826467 |
| TNBC | Apoptosis Assay | 0.01-10μM | 72 h | DMSO | has minimal effect on cell proliferation | 22826467 |
| A498 | Apoptosis Assay | 0.01-10μM | 72 h | DMSO | induces synergistic responses with romidepsin | 22826467 |
| KIJ265T | Apoptosis Assay | 0.01-10μM | 72 h | DMSO | induces synergistic responses with romidepsin | 22826467 |
| MDA-231 | Apoptosis Assay | 0.01-10μM | 72 h | DMSO | induces synergistic responses with romidepsin | 22826467 |
| BT-20 | Apoptosis Assay | 0.01-10μM | 72 h | DMSO | induces synergistic responses with romidepsin | 22826467 |
| U937 | Growth Inhibition Assay | 5-20 μM | 24/48/72 h | induces a decrease in cell viability in a concentration- and time-dependent manner | 22767021 | |
| HL60 | Growth Inhibition Assay | 5-20 μM | 24/48/72 h | induces a decrease in cell viability in a concentration- and time-dependent manner | 22767021 | |
| U937 | Apoptosis Assay | 15 μM | 24/48/72 h | induces cell apoptosis | 22767021 | |
| HL60 | Apoptosis Assay | 15 μM | 24/48/72 h | induces cell apoptosis | 22767021 | |
| LS411N | Apoptosis Assay | 0.5 μM | 72 h | increases Fas mRNA level | 22461695 | |
| MDA-MB-231 | Apoptosis Assay | 10 μM | 48 h | reduces cell viability in a dose-dependent manner | 21887697 | |
| MCF-7 | Apoptosis Assay | 10 μM | 48 h | reduces cell viability in a dose-dependent manner | 21887697 | |
| A375 | Growth Inhibition Assay | 0.5 μM | 1/5/8 d | inhibits proliferation and induces differentiation of melanoma cells | 21796622 | |
| SKMEL1 | Growth Inhibition Assay | 0.5 μM | 1/5/8 d | inhibits proliferation and induces differentiation of melanoma cells | 21796622 | |
| SKMEL3 | Growth Inhibition Assay | 0.5 μM | 1/5/8 d | inhibits proliferation and induces differentiation of melanoma cells | 21796622 | |
| SKMEL28 | Growth Inhibition Assay | 0.5 μM | 1/5/8 d | inhibits proliferation and induces differentiation of melanoma cells | 21796622 | |
| MeWo | Growth Inhibition Assay | 0.5 μM | 1/5/8 d | inhibits proliferation and induces differentiation of melanoma cells | 21796622 | |
| B16 | Growth Inhibition Assay | 0.5 μM | 1/5/8 d | inhibits proliferation and induces differentiation of melanoma cells | 21796622 | |
| Ly 1 | Growth Inhibition Assay | 24 h | IC50=7.3 μM | 21772049 | ||
| Ly 7 | Growth Inhibition Assay | 24 h | IC50=10.7 μM | 21772049 | ||
| Su-DHL6 | Growth Inhibition Assay | 24 h | IC50>20 μM | 21772049 | ||
| Ly 10 | Growth Inhibition Assay | 24 h | IC50>20 μM | 21772049 | ||
| RIVA | Growth Inhibition Assay | 24 h | IC50>20 μM | 21772049 | ||
| Su-DHL2 | Growth Inhibition Assay | 24 h | IC50>20 μM | 21772049 | ||
| Ly 1 | Growth Inhibition Assay | 48 h | IC50=0.34 μM | 21772049 | ||
| Ly 7 | Growth Inhibition Assay | 48 h | IC50=0.025 μM | 21772049 | ||
| Su-DHL6 | Growth Inhibition Assay | 48 h | IC50>20 μM | 21772049 | ||
| Ly 10 | Growth Inhibition Assay | 48 h | IC50=1.8 μM | 21772049 | ||
| RIVA | Growth Inhibition Assay | 48 h | IC50>20 μM | 21772049 | ||
| Su-DHL2 | Growth Inhibition Assay | 48 h | IC50=17.4 μM | 21772049 | ||
| Ly 1 | Growth Inhibition Assay | 72 h | IC50=0.01 μM | 21772049 | ||
| Ly 7 | Growth Inhibition Assay | 72 h | IC50=0.018 μM | 21772049 | ||
| Su-DHL6 | Growth Inhibition Assay | 72 h | IC50=1.6 μM | 21772049 | ||
| Ly 10 | Growth Inhibition Assay | 72 h | IC50=1.2 μM | 21772049 | ||
| RIVA | Growth Inhibition Assay | 72 h | IC50>20 μM | 21772049 | ||
| Su-DHL2 | Growth Inhibition Assay | 72 h | IC50=11.2 μM | 21772049 | ||
| U373-MAGI | Antiviral assay | 0.25 to 8 uM | 2 to 72 hrs | Antiviral activity against VSV-G pseudotyped HIV-1 NL4-3 infected in human U373-MAGI cells assessed as reduction in U5-gag level at 0.25 to 8 uM after 2 to 72 hrs by qPCR method | 27117260 | |
| U373-MAGI | Antiviral assay | 0.25 to 8 uM | 2 to 72 hrs | Antiviral activity against VSV-G pseudotyped HIV-1 NL4-3 infected in human U373-MAGI cells assessed as reduction in gag level at 0.25 to 8 uM after 2 to 72 hrs by qPCR method | 27117260 | |
| Cliquez pour voir plus de données expérimentales sur la lignée cellulaire | ||||||
Informations chimiques, stockage et stabilité
| Poids moléculaire | 228.21 | Formule | C8H12N4O4 |
Stockage (À compter de la date de réception) | |
|---|---|---|---|---|---|
| N° CAS | 2353-33-5 | Télécharger le SDF | Stockage des solutions mères |
|
|
| Synonymes | Deoxycytidine, 5-aza-2'-deoxycytidine, 5-AZA-dC, 5-aza-CdR,NSC 127716 | Smiles | C1C(C(OC1N2C=NC(=NC2=O)N)CO)O | ||
Solubilité
|
In vitro |
DMSO
: 45 mg/mL
(197.18 mM)
Water : 22.5 mg/mL Ethanol : Insoluble |
Calculateur de molarité
|
In vivo |
|||||
Calculateur de formulation in vivo (Solution claire)
Étape 1 : Saisir les informations ci-dessous (Recommandé : Un animal supplémentaire pour tenir compte des pertes pendant lexpérience)
Étape 2 : Saisir la formulation in vivo (Ceci est seulement le calculateur, pas la formulation. Veuillez nous contacter dabord sil ny a pas de formulation in vivo dans la section Solubilité.)
Résultats du calcul :
Concentration de travail : mg/ml;
Méthode de préparation du liquide maître DMSO : mg médicament prédissous dans μL DMSO ( Concentration du liquide maître mg/mL, Veuillez nous contacter dabord si la concentration dépasse la solubilité du DMSO du lot de médicament. )
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouterμL PEG300, mélanger et clarifier, puis ajouterμL Tween 80, mélanger et clarifier, puis ajouter μL ddH2O, mélanger et clarifier.
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouter μL Huile de maïs, mélanger et clarifier.
Note : 1. Veuillez vous assurer que le liquide est clair avant dajouter le solvant suivant.
2. Assurez-vous dajouter le(s) solvant(s) dans lordre. Vous devez vous assurer que la solution obtenue, lors de lajout précédent, est une solution claire avant de procéder à lajout du solvant suivant. Des méthodes physiques telles que le vortex, les ultrasons ou le bain-marie chaud peuvent être utilisées pour faciliter la dissolution.
Mécanisme daction
| Targets/IC50/Ki |
DNA methylation
(HL-60, KG1a cells) |
|---|---|
| In vitro |
La Decitabine inhibe la croissance cellulaire de manière dose- et temps-dépendante avec des IC50 d'environ 438 nM et 43,8 nM pour des expositions de 72 heures et 96 heures dans les cellules leucémiques HL-60 et KG1a, respectivement. Une étude récente montre que ce composé présente une activité anti-proliférative et pro-apoptotique élevée contre le lymphome anaplasique à grandes cellules (LAGC), et inhibe l'incorporation de [3H]–thymidine dans les cellules KARPAS-299 avec une EC50 de 0,49 μM. |
| Essai kinase |
dosage de synthèse d'ADN
|
|
Le taux de synthèse de l'ADN est mesuré par l'incorporation de thymidine radioactive dans l'ADN. Les cellules HL-60 et KG1a sont suspendues dans 2 mL de milieu RPMI contenant 10% de sérum fœtal dans des boîtes de 6 puits (35 mm de diamètre) et incubées avec différentes concentrations de médicaments correspondants pendant 48 heures (les médicaments sont ajoutés simultanément). À 48 heures, 0,5 μCi de [3H] thymidine (6,7 Ci/mmol) est ajouté à chaque puits et incubé pendant 24 heures supplémentaires. Les cellules sont placées sur des filtres en fibre de verre GF/C (2,4 cm de diamètre), lavées avec du NaCl froid à 0,9%, de l'acide trichloroacétique froid à 5% et de l'éthanol. Les filtres contenant l'ADN sont ensuite séchés, placés dans du liquide de scintillation EcoLite (ICN) et la radioactivité mesurée à l'aide d'un compteur à scintillation Beckman LS 6000IC. L'IC50 est définie comme la concentration de médicament qui inhibe 50% de la synthèse d'ADN des lignées de cellules leucémiques à partir de la courbe dose-réponse.
|
|
| In vivo |
Dans un modèle de xénogreffe murin ALK+ KARPAS-299, la Decitabine à une dose de 2,5 mg/kg entraîne une augmentation de l'apoptose et une réduction de la prolifération des cellules tumorales, et résulte également en une déméthylation du suppresseur de tumeur p16INK4A. |
Références |
|
Applications
| Méthodes | Biomarqueurs | Images | PMID |
|---|---|---|---|
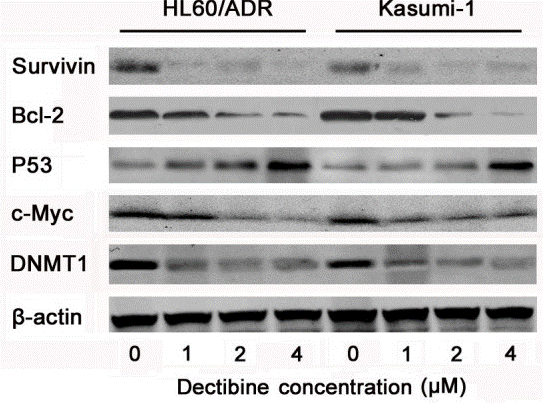
| Western blot | Survivin / Bcl-2 / p53 / c-Myc / DNMT1 p-AKT / AKT / p-GSK3β / GSK3β / p-Myc / Myc / p-P70 / P70 / p-4EBP-1 / 4EBP-1 / PTEN phospho-p38 / p38 / phospho-NFκB / NFκB p-JAK1 / JAK1 / p-JAK2 / JAK2 / p-STAT3 / STAT3 E-cadherin / N-cadherin / Snail / MMP-2 / MMP-9 / Bcl-2 / Bax |
|
26384351 |
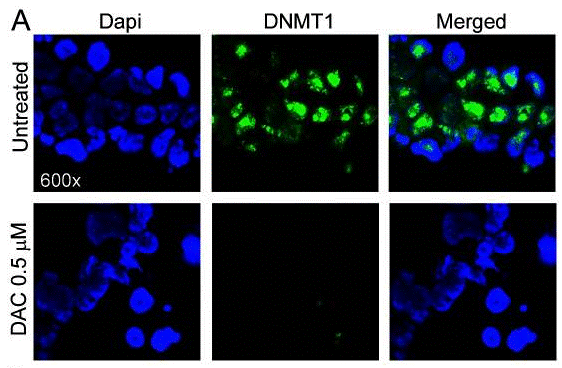
| Immunofluorescence | DNMT1 E-cadherin / MMP-9 |
|
21303982 |
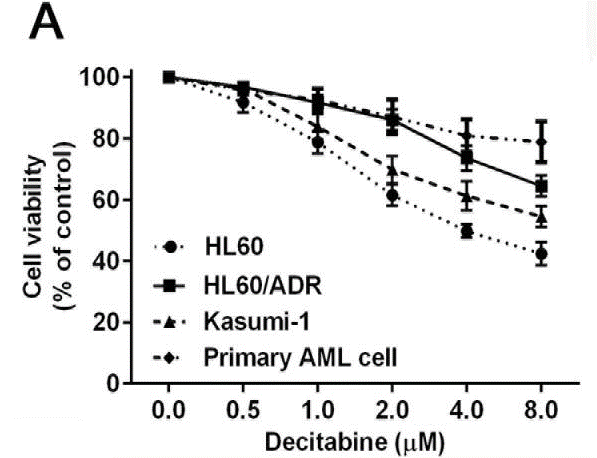
| Growth inhibition assay | Cell viability |
|
26384351 |
Informations sur lessai clinique
(données du https://clinicaltrials.gov, mis à jour le 2024-05-22)
| Numéro NCT | Recrutement | Conditions | Promoteur/Collaborateurs | Date de début | Phases |
|---|---|---|---|---|---|
| NCT06291285 | Not yet recruiting | Healthy Volunteers |
Novo Nordisk A/S |
February 27 2024 | Phase 1 |
| NCT05960773 | Recruiting | Mesothelioma|Malignant Mesothelioma (MM)|Early-stage Mesothelioma|Subclinical Mesothelioma|BRCA1-Associated Protein-1 (BAP1) Mutations|Early-stage BAP1-associated Malignancies |
National Cancer Institute (NCI)|National Institutes of Health Clinical Center (CC) |
January 31 2024 | Phase 2 |
| NCT05835011 | Terminated | Myelodysplastic Syndromes |
Astex Pharmaceuticals Inc. |
July 14 2023 | Phase 2 |
| NCT05816356 | Recruiting | Healthy |
EpiDestiny Inc.|Worldwide Clinical Trials |
March 24 2023 | Phase 1 |
Support technique
Tel: +1-832-582-8158 Ext:3
Si vous avez dautres questions, veuillez laisser un message.
Foire aux questions
Question 1:
Is it a racemic mixture or a monomer?
Réponse :
Its S1200 is R form.
