pour la recherche uniquement
3-Methyladenine (3-MA) Inhibiteur d'Autophagy/PI3K
Réf. CatalogueS2767
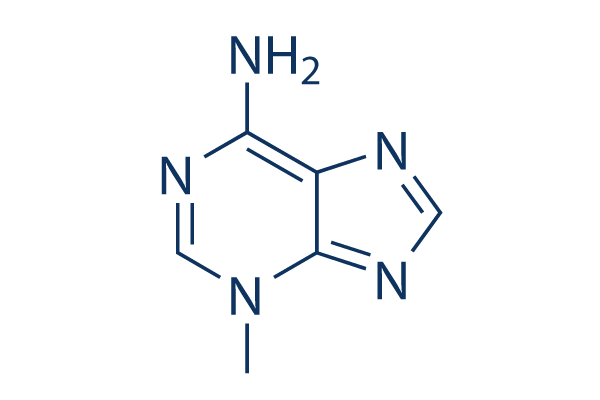
Structure chimique
Poids moléculaire: 149.15
Contrôle Qualité
Produits souvent utilisés avec 3-Methyladenine (3-MA)
| Cibles apparentées | Akt mTOR GSK-3 ATM/ATR DNA-PK AMPK PDPK1 PTEN PP2A PDK |
|---|---|
| Autre PI3K Inhibiteurs | GDC-0077 (Inavolisib) SAR405 Quercetin (Sophoretin) LY294002 XL147 analogue Tersolisib (STX-478) Buparlisib (BKM120) 740 Y-P (PDGFR 740Y-P) GO-203 TFA Eganelisib (IPI-549) |
Culture cellulaire, traitement et concentration de travail
| Lignées cellulaires | Type dessai | Concentration | Temps dincubation | Formulation | Description de lactivité | PMID |
|---|---|---|---|---|---|---|
| K562 | Function Assay | 10mM | 1h | decreases the expression of LC3-II and the formation of autophagosomes | 21864037 | |
| Jurkat | Function Assay | 10mM | 1h | decreases the expression of LC3-II and the formation of autophagosomes | 21864037 | |
| HeLa | Cytotoxicity Assay | 2mM | 24h | inhibites the cytotoxicity of silibinin to HeLa cells. | 21875385 | |
| PC12/TetOn | Function Assay | 0.1/1mM | 18h | leads to α-syn(WT) accumulation, toxicity, and oligomer formation | 21906659 | |
| RMPI8226 | Function Assay | 5mM | 1h | suppresses the level of autophagy under nutrient depletion | 21915620 | |
| MCF-7 | Function Assay | 10mM | 48h | blocks autophagy induced by bortezomib | 21931937 | |
| HBx | Apoptosis Assay | 10mM | 48h | DMSO | increases cell death | 22020078 |
| Marc-145 | Function Assay | 5mM | 12/24/36h | reduces the PRRSV titers and the protein expression | 22119900 | |
| U937 | Function Assay | 2mM | 12h | decreases the autophagy ratio | 22155150 | |
| BGC-823 | Function Assay | 5mM | 2h | inhibits the rate of autophagic cells | 22322152 | |
| A549 | Function Assay | 0.1mM | 24h | suppresses SU11274-induced cell death | 22466960 | |
| pDCs | Function Assay | 10mM | 0.5h | reduces the induction of IFN-α by ssRNA40 | 22396599 | |
| HeLa | Apoptosis Assay | 5mM | 24h | induces caspase-dependent cell death | 22545128 | |
| U251 | Apoptosis Assay | 5mM | 24h | increases S1-induced cell death | 22579788 | |
| MCF-7 | Apoptosis Assay | 0.1mM | 6h | enhances sirtinol-induced apoptosis | 22751989 | |
| PC-3 | Apoptosis Assay | 2mM | 2h | increases ORI-induced cell death | 22745580 | |
| HCT116 | Apoptosis Assay | 5mM | 24h | DMSO | enhances apigenin-induced cell death | 24626522 |
| U2OS | Growth Inhibition Assay | 10mM | 24h | intensifies the growth inhibition induced by Dox | 24639013 | |
| A2780cp | Apoptosis Assay | 2.5mM | 1h | ddH2O | enhances cisplatin-induced cell death | 24817946 |
| HepG2 | Function Assay | 5mM | 4h | increases cellular levels of HL | 24713587 | |
| Microglia | Apoptosis Assay | 5mM | 24h | decreases hypoxia-induced cell death | 24818601 | |
| MDA-MB 231 | Apoptosis Assay | 5mM | 0.5h | modulates Tocomin® induced apoptosis | 24830781 | |
| PANC-1 | Apoptosis Assay | 1mM | 48h | DMSO | enhances bortezomib-induced cell viability loss | 24842158 |
| MDA-MB-231 | Function Assay | 2mM | 48h | promotes TM-induced cell death | 24970676 | |
| MDA-MB-231 | Function Assay | 2mM | 24h | inhibits autophagy induced by TM | 24970676 | |
| MCF-7 | Function Assay | 2mM | 48h | promotes TM-induced cell death | 24970676 | |
| MCF-7 | Function Assay | 2mM | 24h | inhibits autophagy induced by TM | 24970676 | |
| HepG2 | Apoptosis Assay | 3mM | 5h | reduces cell apoptosis induced by QDs | 22836595 | |
| HeLa | Apoptosis Assay | 10mM | 2h | decreases cell viability co-treatment with PEI | 23000135 | |
| SK-HEP-1 | Apoptosis Assay | 10mM | 1h | protects against autophagy and induces apoptosis in bufalin-treated cells | 22858649 | |
| MDA-MB231 | Function Assay | 5mM | 1h | increases resveratrol-mediated caspase activation and cell death | 23088850 | |
| PaCa44 | Apoptosis Assay | 2.5mM | 1h | reduces genipin-mediated apoptosis | 23124112 | |
| T-47D | Function Assay | 10mM | 2h | inhibits autophagy process and increases rapamycin induced apoptosis | 23300026 | |
| GTL-16 | Apoptosis Assay | 5mM | 24h | reduces cell viability as compared to cells treated with MET inhibitors | 23313490 | |
| U251MG | Function Assay | 3mM | 1h | suppresses LC3-II protein expression | 23338618 | |
| T24 | Function Assay | 10mM | 1h | reduces the cleavage of LC3 after baicalin treatment | 23354080 | |
| HUVECs | Function Assay | 3mM | 24h | blocks the protective effect of resveratrol by inhibiting autophagy | 23358928 | |
| MCF-7 | Function Assay | 5mM | 24h | inhibits starvation-induced autophagy | 23395679 | |
| Hela | Function Assay | 5mM | 24h | inhibits starvation-induced autophagy | 23395679 | |
| OR6 | Function Assay | 10mM | 72h | suppresses HCV replication and formation of autophagosomes | 23395875 | |
| HT-29 | Function Assay | 1mM | 48/96h | inhibits AMPK induces autophagic cell death | 23508272 | |
| SH-SY5Y | Cytotoxicity Assay | 5mM | 24h | increases PCN toxicity | 23525265 | |
| Saos-2 | Apoptosis Assay | 1mM | 96h | increases cell death induced by PCX | 23563171 | |
| 1321N1 | Cytotoxicity Assay | 5mM | 24h | protects cell against PCN-induced toxicity | 23525265 | |
| A2780 | Apoptosis Assay | 5mM | 24h | converts FTY720 with CDDP into an additive effect towards killing ovarian cancer cells | 23592281 | |
| OV2008 | Apoptosis Assay | 5mM | 24h | converts FTY720 with CDDP into an additive effect towards killing ovarian cancer cells | 23592281 | |
| PC12 | Function Assay | 10mM | 24h | water | inhibits chymotrypsin-like proteasomal activity. | 23603979 |
| SH-SY5Y | Apoptosis Assay | 5mM | 1h | abolishes celastrol neuroprotective effect | 23619395 | |
| SH-SY5Y | Function Assay | 1mM | 24h | inhibits the autophagy induced by TOCP | 23743148 | |
| HepG2 | Function Assay | 10mM | 24h | inhibits siTIGAR- and HBSS-induced autophagy | 23817040 | |
| HeLa | Function Assay | 10mM | 2h | suppresses LC3 II expressison | 23864738 | |
| HONE-1 | Function Assay | 5mM | 1h | represses 6r-mediated ROS production | 23892358 | |
| MCF7 | Function Assay | 5mM | 24h | increases CuO induced cell death | 23962629 | |
| HO8910 | Apoptosis Assay | 10mM | 12h | enhances B19-induced apoptosi | 23983610 | |
| SMMC-7721 | Apoptosis Assay | 5mM | 24h | attenuates TNF-α protection against serum starvation-mediated apoptosis | 24066693 | |
| Hep3B | Apoptosis Assay | 5mM | 24h | attenuates TNF-α protection against serum starvation-mediated apoptosis | 24066693 | |
| H460 | Function Assay | 10mM | 4h | increases cisplatin-induced cell death | 24173208 | |
| A549 | Function Assay | 10mM | 4h | inhibits autophagy induced by irradiation | 24142735 | |
| H1299 | Function Assay | 10mM | 4h | increases cisplatin-induced cell death | 24173208 | |
| WiDr | Function Assay | 10mM | 1h | inhibits PCBL-induced LC3 II expression | 24190489 | |
| LoVo | Apoptosis Assay | 5mM | 48h | enhances DCA-induced apoptosis. | 24201812 | |
| HepG2 E47 | Function Assay | 2.5mM | 48h | increases the toxicity of AA, BSO, and CCl4 | 24273738 | |
| RKO | Function Assay | 2mM | 1h | DMSO | enhances cell death by geldanamycin | 24291777 |
| Hep3B | Apoptosis Assay | 2mM | 12h | DMSO | inhibits AZD8055-induced cell death | 24297300 |
| ACHN-5968 | Apoptosis Assay | 5mM | 3h | enhances paclitaxel-mediated apoptosis | 24305604 | |
| Huh7 | Apoptosis Assay | 2mM | 12h | DMSO | inhibits AZD8055-induced cell death | 24297300 |
| UOK257 | Apoptosis Assay | 5mM | 3h | enhances paclitaxel-mediated apoptosis | 24305604 | |
| ECSCs | Apoptosis Assay | 10mM | 4h | decreases rapamycin-treated apoptosis | 24319109 | |
| MCF-7 | Function Assay | 10mM | 24h | inhibits the autophagy induced by chemotherapy drugs | 24315578 | |
| SGC-7901 | Apoptosis Assay | 2mM | 1h | increases CA-4 induced apoptosis | 24321340 | |
| SMMC-7721 | Apoptosis Assay | 2mM | 1h | increases CA-4 induced apoptosis | 24321340 | |
| T24 | Apoptosis Assay | 5mM | 1.5h | potentiates celecoxib-induced apoptosis | 24349176 | |
| NTUB1 | Apoptosis Assay | 5mM | 1.5h | potentiates celecoxib-induced apoptosis | 24349176 | |
| MG-63 | Apoptosis Assay | 10mM | 12h | enhances DP-induced apoptosis | 24358301 | |
| MG-63 | Apoptosis Assay | 0.5/1mM | 32h | enhances salinomycin-induced cell apoptosis | 24358342 | |
| MG-63 | Function Assay | 0.5/1mM | 48h | induces salinomycin-induced cell viability loss | 24358342 | |
| U2OS | Function Assay | 0.5/1mM | 48h | induces salinomycin-induced cell viability loss | 24358342 | |
| HGC-27 | Function Assay | 10mM | 1h | inhibits the cell viability loss by RAD001 or MK-2206 | 24416349 | |
| HCT116 | Apoptosis Assay | 5mM | 24h | enhances the apoptosis induced by apigenin | 24626522 | |
| A549 | Apoptosis Assay | 10mM | 48h | accelerates the reduction of cell viability induced by PTX | 24626722 | |
| Saos-2 | Apoptosis Assay | 10mM | 24h | intensifies the growth inhibition of the U2OS cells induced by Dox | 24639013 | |
| U2OS | Apoptosis Assay | 10mM | 24h | intensifies the growth inhibition of the U2OS cells induced by Dox | 24639013 | |
| HepG2 | Function Assay | 5mM | 4h | increases HL release | 24713587 | |
| A549 | Apoptosis Assay | 5mM | 48h | decreases the percentage of cell death and expression levels of caspase-3, Beclin-1 and LC3-II | 24706303 | |
| A2780cp | Apoptosis Assay | 2.5mM | 1h | ddH2O | enhances cisplatin-induced cell death | 24817946 |
| Microglia | Apoptosis Assay | 5mM | 24h | decreases hypoxia-induced cell death | 24818601 | |
| HT-29 | Apoptosis Assay | 1mM | 48h | DMSO | enhances bortezomib-induced cell viability loss | 24842158 |
| MDR | Apoptosis Assay | 10mM | 6h | strengthens the power of anticancer drugs | 25019701 | |
| H157 | Function Assay | 5mM | 2h | suppresses SPC induced accumulation of LC3-II | 25285628 | |
| A549 | Function Assay | 5mM | 2h | suppresses SPC induced accumulation of LC3-II | 25285628 | |
| A2780cp | Growth Inhibition Assay | 1mM | 1h | increases cisplatin-induced cell death | 25322694 | |
| NBL-W-S | Apoptosis Assay | 1mM | 6h | increases cell apoptosis induced by GANT-61 | 25323222 | |
| NBL-W-S | Growth Inhibition Assay | 1mM | 6h | enhances GANT-61 toxicity | 25323222 | |
| A549 | Apoptosis Assay | 5mM | 2h | DMSO | inhibits BDMC-induced apoptotic cell death | 25716561 |
| 95D | Apoptosis Assay | 5mM | 2h | DMSO | inhibits BDMC-induced apoptotic cell death | 25716561 |
| A549 | Growth Inhibition Assay | 3mM | 2h | DMSO | reduces growth inhibitory effect of BDMC | 25716561 |
| 95D | Growth Inhibition Assay | 3mM | 2h | DMSO | reduces growth inhibitory effect of BDMC | 25716561 |
| Nara-H | Growth Inhibition Assay | 5mM | 48h | enhances temsirolimusmediated suppression of Nara-H cell proliferation | 21805033 | |
| HUVECs | Function Assay | 10mM | 0.5h | decreases the AGE-BSAinduced autophagy leve | 21468592 | |
| HepG2 | Apoptosis Assay | 2mM | 1h | enhances radiation-induced cell death | 21453691 | |
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| Rh30 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh30 cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| Cliquez pour voir plus de données expérimentales sur la lignée cellulaire | ||||||
Informations chimiques, stockage et stabilité
| Poids moléculaire | 149.15 | Formule | C6H7N5 |
Stockage (À compter de la date de réception) | 3 years -20°C powder |
|---|---|---|---|---|---|
| N° CAS | 5142-23-4 | Télécharger le SDF | Stockage des solutions mères | Les solutions sont instables. Préparer des solutions fraîches ou acheter des formats petits et préemballés. Reconditionner dès réception. | |
| Synonymes | NSC 66389 | Smiles | CN1C=NC(=N)C2=C1N=CN2 | ||
Solubilité
|
In vitro |
DMSO
: 10 mg/mL
(67.04 mM)
Réchauffé avec un bain-marie à 50°C;
Ultrasonifié;
Ethanol : 10 mg/mL Water : 4 mg/mL |
Calculateur de molarité
|
In vivo |
|||||
Calculateur de formulation in vivo (Solution claire)
Étape 1 : Saisir les informations ci-dessous (Recommandé : Un animal supplémentaire pour tenir compte des pertes pendant lexpérience)
Étape 2 : Saisir la formulation in vivo (Ceci est seulement le calculateur, pas la formulation. Veuillez nous contacter dabord sil ny a pas de formulation in vivo dans la section Solubilité.)
Résultats du calcul :
Concentration de travail : mg/ml;
Méthode de préparation du liquide maître DMSO : mg médicament prédissous dans μL DMSO ( Concentration du liquide maître mg/mL, Veuillez nous contacter dabord si la concentration dépasse la solubilité du DMSO du lot de médicament. )
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouterμL PEG300, mélanger et clarifier, puis ajouterμL Tween 80, mélanger et clarifier, puis ajouter μL ddH2O, mélanger et clarifier.
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouter μL Huile de maïs, mélanger et clarifier.
Note : 1. Veuillez vous assurer que le liquide est clair avant dajouter le solvant suivant.
2. Assurez-vous dajouter le(s) solvant(s) dans lordre. Vous devez vous assurer que la solution obtenue, lors de lajout précédent, est une solution claire avant de procéder à lajout du solvant suivant. Des méthodes physiques telles que le vortex, les ultrasons ou le bain-marie chaud peuvent être utilisées pour faciliter la dissolution.
Mécanisme daction
| Targets/IC50/Ki |
Autophagy
Vps34
(HeLa cells) 25 μM
PI3Kγ
(HeLa cells) 60 μM
|
|---|---|
| In vitro |
La légère préférence pour la prévention de Vps34 par la 3-Methyladenine (3-MA) provient probablement d'un cycle hydrophobe spécifique à Vps34, qui entoure le groupe 3-méthyle de ce composé. Il a été rapporté qu'il provoque la mort des cellules cancéreuses dans des conditions normales et de famine, et qu'il pourrait également supprimer la migration et l'invasion cellulaires indépendamment de sa capacité à inhiber l'autophagy, ce qui implique qu'il possède des fonctions autres que la suppression de l'autophagy. Ce composé déclenche une mort cellulaire dépendante de la caspase qui est indépendante de l'inhibition de l'autophagy. Le traitement avec 5 mM de celui-ci réduit le pourcentage de cellules HeLa privées de glucose affichant des points GFP-LC3 à 23 %. Les niveaux de LC3-I augmentent et les niveaux de LC3-II diminuent entre 12 et 48 heures dans les cellules traitées avec 3-MA. La conversion de LC3-I en LC3-II est supprimée par le composé. Le traitement des cellules HeLa avec 2,5 mM ou 5 mM pendant un jour n'affecte pas la viabilité cellulaire, tandis que le traitement avec 10 mM pendant un jour provoque une diminution de 25,0 % de la viabilité cellulaire. Le traitement des cellules avec 2,5, 5 ou 10 mM pendant deux jours entraîne une diminution de la viabilité de 11,5 %, 38,0 % et 79,4 %, respectivement. Il diminue la viabilité cellulaire de manière dépendante du temps et de la dose, et raccourcit significativement la durée de l'arrêt de la prométaphase induit par le nocodazole. La suppression de l'autophagy par le 3-MA inhibe la mort cellulaire induite par le SU11274. Un traitement prolongé avec celui-ci (jusqu'à 9 heures) induit une conversion significative de LC3 I en II dans les MEF de type sauvage. Un traitement prolongé avec le 3-MA, mais pas la wortmannine, augmente nettement la ponctuation/agrégation de GFP-LC3. Sa conversion de LC3 induite et la libération de GFP libre sont dépendantes d'ATG7. Le traitement avec celui-ci conduit à une augmentation évidente du niveau de protéine p62. Le composé augmente le niveau de p62 même dans les MEF Atg5-/-- ainsi que dans les cellules avec délétion d'ATG5 médiatisée par la DOX. Il inhibe les PI3K de classe I et de classe III selon différents schémas temporels. Sa conversion de LC3 I en LC3 II induite est considérablement compromise dans les cellules Tsc2-/-- par rapport aux cellules de type sauvage. Ce composé perturbe la fonction anti-autophagique du complexe mTOR 1. |
| Essai kinase |
Essai de dégradation des protéines
|
|
Les cellules HeLa sont radiomarquées pendant 24 heures avec 0,05 mCi/mL de l-[U- 14C]valine. À la fin de la période de marquage, les cellules sont rincées trois fois avec du PBS. Les cellules sont incubées pendant les temps désignés dans un milieu complet ou EBSS avec ou sans la présence de 10 mM de 3-Methyladenine (3-MA).
|
|
| In vivo |
La 3-Methyladenine (3-MA) bloque l'autophagy par son effet sur la phosphatidylinositol 3-kinase (PI3K) de classe III. Le traitement avec ce composé ne modifie pas le degré d'hémorragie par rapport au groupe hémorragie sous-arachnoïdienne (HSA). Son prétraitement aggrave significativement les symptômes neurologiques par rapport au groupe HSA + véhicule. L'autophagy est diminuée lors de son application. Inversement, la caspase-3 clivée est nettement régulée à la hausse dans le groupe HSA + 3-MA. Conformément à la régulation à la hausse de l'expression de la caspase-3 clivée, le nombre de cellules TUNEL-positives dans le cortex droit est significativement augmenté dans le groupe HSA + 3-MA par rapport au groupe HSA + véhicule. |
Références |
|
Applications
| Méthodes | Biomarqueurs | Images | PMID |
|---|---|---|---|
| Western blot | α-SMA / TGF-β / LC-3BI / LC-3B II / Beclin-1 / NF-κB p65 caspase-3 / caspase-9 / PARP VEGF APP / BACE1 / ADAM17 / Presenilin 1 / Presenilin 2 / Nicastrin / APH-1 / Pen-2 / LC3-1 / LC3-2 |
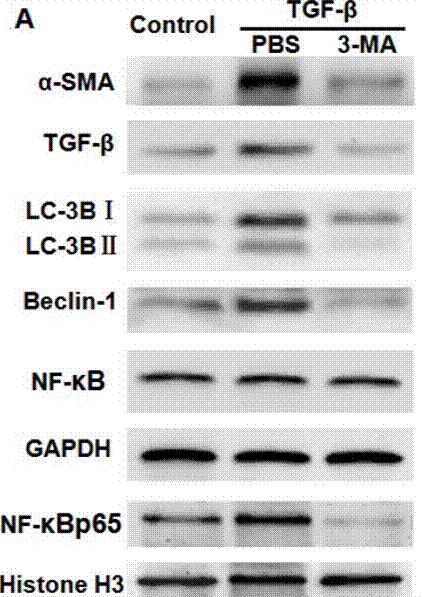
|
29296191 |
| Immunofluorescence | LC3 / Hif-α / COX2 |
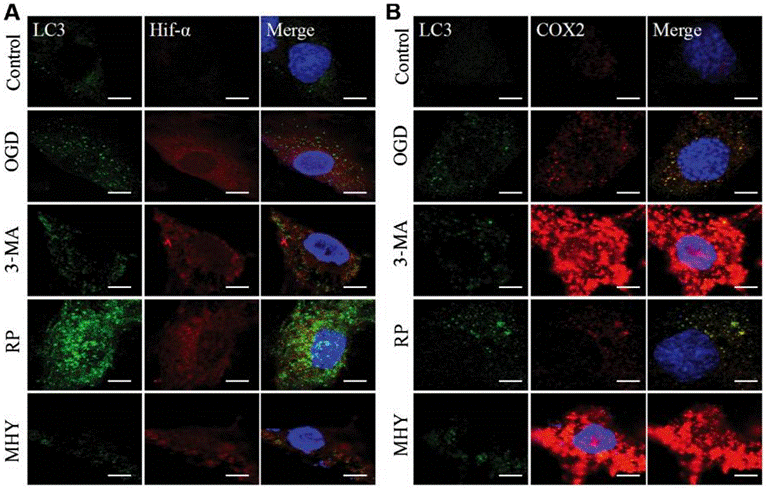
|
29039446 |
| Growth inhibition assay | Cell viability |
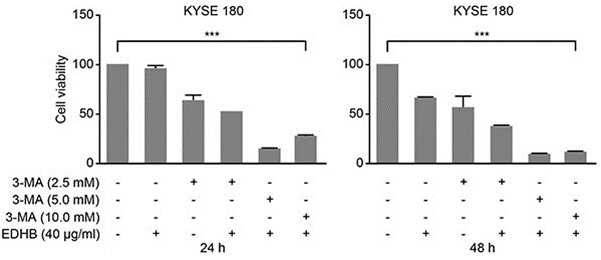
|
26934124 |
Support technique
Tel: +1-832-582-8158 Ext:3
Si vous avez dautres questions, veuillez laisser un message.
Foire aux questions
Question 1:
I'm also wondering whether it can be dissolved in water, or maybe something like culture medium, normal saline solution to form 10mM solution.
Réponse :
As the reference (http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal. pone.0035665), it was found to inhibit autophagy at concentrations ranging from 1 to 10 mM and was directly dissolved into the culture medium at the indicated concentrations. And we tested the solubility of S2767, and found its solubility in DMEM is 31 mg/mL at about 40°C.
