pour la recherche uniquement
Tetraethylthiuram disulfide (Disulfiram) inhibiteur de l'ALDH
Réf. CatalogueS1680
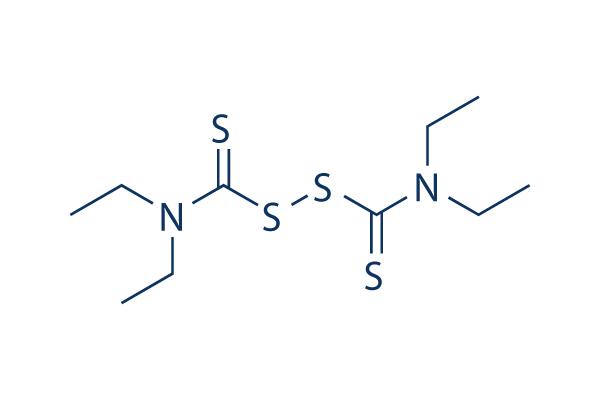
Structure chimique
Poids moléculaire: 296.54
Contrôle Qualité
Culture cellulaire, traitement et concentration de travail
| Lignées cellulaires | Type dessai | Concentration | Temps dincubation | Formulation | Description de lactivité | PMID |
|---|---|---|---|---|---|---|
| insect cells | Function assay | 30 mins | Inhibition of recombinant human LOXL4 expressed in baculovirus infected insect cells using diaminopentane as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by fluorimetric method, IC50 = 0.059 μM. | 30098867 | ||
| CHO | Function assay | 30 mins | Inhibition of recombinant human LOXL3 expressed in CHO cells using diaminopentane as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by fluorimetric method, IC50 = 0.093 μM. | 30098867 | ||
| MCF7 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF7 cells expressing BCA2 and estrogen receptor after 72 hrs by MTT assay, IC50 = 0.1 μM. | 20222671 | ||
| NS0 | Function assay | 30 mins | Inhibition of recombinant LOXL2 (unknown origin) expressed in NS0 cells using diaminopentane as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by fluorimetric method, IC50 = 0.15 μM. | 30098867 | ||
| T47D | Antiproliferative assay | 72 hrs | Antiproliferative activity against human T47D cells expressing BCA2 and estrogen receptor after 72 hrs by MTT assay, IC50 = 0.17 μM. | 20222671 | ||
| MDA-MB-231 | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MDA-MB-231 cells expressing BCA2 and ERalpha after 72 hrs by MTT assay, IC50 = 0.32 μM. | 20222671 | ||
| HEK293 | Function assay | 30 mins | Inhibition of recombinant human LOX expressed in HEK293 cells using diaminopentane as substrate preincubated for 30 mins followed by substrate addition measured after 1 hr by fluorimetric method, IC50 = 0.32 μM. | 30098867 | ||
| CHO | Function assay | Agonist activity at human TRPA1 channel expressed in CHO cells assessed as increase in intracellular calcium levels, EC50 = 3 μM. | 20356305 | |||
| CEM-SS | Antiviral assay | Antiviral activity against HIV-1 in CEM-SS cells using XXT assay, IC50 = 3.9 μM. | 9207937 | |||
| MCF10A | Antiproliferative assay | 72 hrs | Antiproliferative activity against human MCF10A cells after 72 hrs by MTT assay, IC50 = 10 μM. | 20222671 | ||
| HepG2 | Cytotoxicity assay | 72 hrs | Cytotoxicity against human HepG2 cells assessed as reduction in cell viability after 72 hrs by MTT assay, IC50 = 38 μM. | 29571571 | ||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for TC32 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SJ-GBM2 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for A673 cells | 29435139 | |||
| SK-N-MC | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-MC cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-37 cells | 29435139 | |||
| NB-EBc1 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB-EBc1 cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for U-2 OS cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Saos-2 cells | 29435139 | |||
| SK-N-SH | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for SK-N-SH cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for NB1643 cells | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for LAN-5 cells | 29435139 | |||
| BT-12 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for BT-12 cells | 29435139 | |||
| Rh18 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh18 cells | 29435139 | |||
| OHS-50 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for OHS-50 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for RD cells | 29435139 | |||
| MG 63 (6-TG R) | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for MG 63 (6-TG R) cells | 29435139 | |||
| fibroblast cells | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for control Hh wild type fibroblast cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Primary screen for Rh41 cells | 29435139 | |||
| NB1643 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for NB1643 cells | 29435139 | |||
| A673 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for A673 cells) | 29435139 | |||
| LAN-5 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for LAN-5 cells | 29435139 | |||
| DAOY | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for DAOY cells | 29435139 | |||
| SJ-GBM2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for SJ-GBM2 cells | 29435139 | |||
| BT-37 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for BT-37 cells | 29435139 | |||
| TC32 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for TC32 cells | 29435139 | |||
| U-2 OS | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for U-2 OS cells | 29435139 | |||
| Rh41 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Rh41 cells | 29435139 | |||
| RD | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for RD cells | 29435139 | |||
| Saos-2 | qHTS assay | qHTS of pediatric cancer cell lines to identify multiple opportunities for drug repurposing: Confirmatory screen for Saos-2 cells | 29435139 | |||
| Cliquez pour voir plus de données expérimentales sur la lignée cellulaire | ||||||
Informations chimiques, stockage et stabilité
| Poids moléculaire | 296.54 | Formule | C10H20N2S4 |
Stockage (À compter de la date de réception) | |
|---|---|---|---|---|---|
| N° CAS | 97-77-8 | Télécharger le SDF | Stockage des solutions mères |
|
|
| Synonymes | NSC 190940, Tetraethylthiuram disulfide, TETD | Smiles | CCN(CC)C(=S)SSC(=S)N(CC)CC | ||
Solubilité
|
In vitro |
DMSO
: 59 mg/mL
(198.96 mM)
Ethanol : 59 mg/mL Water : Insoluble |
Calculateur de molarité
|
In vivo |
|||||
Calculateur de formulation in vivo (Solution claire)
Étape 1 : Saisir les informations ci-dessous (Recommandé : Un animal supplémentaire pour tenir compte des pertes pendant lexpérience)
Étape 2 : Saisir la formulation in vivo (Ceci est seulement le calculateur, pas la formulation. Veuillez nous contacter dabord sil ny a pas de formulation in vivo dans la section Solubilité.)
Résultats du calcul :
Concentration de travail : mg/ml;
Méthode de préparation du liquide maître DMSO : mg médicament prédissous dans μL DMSO ( Concentration du liquide maître mg/mL, Veuillez nous contacter dabord si la concentration dépasse la solubilité du DMSO du lot de médicament. )
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouterμL PEG300, mélanger et clarifier, puis ajouterμL Tween 80, mélanger et clarifier, puis ajouter μL ddH2O, mélanger et clarifier.
Méthode de préparation de la formulation in vivo : Prendre μL DMSO liquide maître, puis ajouter μL Huile de maïs, mélanger et clarifier.
Note : 1. Veuillez vous assurer que le liquide est clair avant dajouter le solvant suivant.
2. Assurez-vous dajouter le(s) solvant(s) dans lordre. Vous devez vous assurer que la solution obtenue, lors de lajout précédent, est une solution claire avant de procéder à lajout du solvant suivant. Des méthodes physiques telles que le vortex, les ultrasons ou le bain-marie chaud peuvent être utilisées pour faciliter la dissolution.
Mécanisme daction
| Targets/IC50/Ki |
ALDH1
(Cell-free assay) 0.15 μM
ALDH2
(Cell-free assay) 1.45 μM
|
|---|---|
| In vitro |
Le complexe Disulfiram-cuivre inhibe puissamment l'activité protéasomale dans les cellules de cancer du sein MDA-MB-231 et MCF10DCIS.com cultivées, mais pas les cellules normales et immortalisées MCF-10A, avant l'induction de la mort cellulaire apoptotique du cancer. Ce composé (DS), un médicament anti-alcoolisme cliniquement utilisé, inhibe fortement l'activité constitutive et induite par le 5-FU de NF-kappaB de manière dose-dépendante. Il inhibe la translocation nucléaire de NF-kappaB et l'activité de liaison à l'ADN, mais n'a aucun effet sur la dégradation d'IkappaBalpha induite par le 5-FU. Cette substance chimique améliore significativement l'effet apoptotique du 5-FU sur les lignées cellulaires DLD-1 et RKO(WT) et potentialise synergiquement la cytotoxicité du 5-FU pour les deux lignées cellulaires. Il abolit également efficacement la chimiorésistance au 5-FU dans une lignée cellulaire résistante au 5-FU H630(5-FU) in vitro. L'oseltamivir diminue le nombre de cellules viables, et l'ajout de CuCl(2) améliore significativement la mort cellulaire induite par le DSF à moins de 10% du contrôle. Ce composé, administré aux cellules de mélanome en combinaison avec Cu2+ ou Zn2+, diminue l'expression de la cycline A et réduit la prolifération in vitro à des concentrations plus faibles que le disulfiram seul. |
| In vivo |
Disulfiram inhibe significativement la croissance tumorale (de 74 %), associée à une inhibition protéasomale in vivo (mesurée par la diminution des niveaux d'activité protéasomale du tissu tumoral et l'accumulation de protéines ubiquitinylées et de substrats naturels du protéasome p27 et Bax) et à l'induction de l'apoptose (comme le montrent l'activation de la caspase et la formation de noyaux apoptotiques) chez des souris porteuses de xénogreffes tumorales MDA-MB-231. Ce composé bloque la pompe d'extrusion de la P-glycoprotéine, inhibe le facteur de transcription nucléaire factor-kappaB, sensibilise les tumeurs à la chimiothérapie, réduit l'angiogenèse et inhibe la croissance tumorale chez les souris. Il inhibe la croissance et l'angiogenèse dans les mélanomes transplantés chez des souris immunodéficientes combinées sévères, et ces effets sont potentialisés par une supplémentation en Zn2+. |
Références |
|
Informations sur lessai clinique
(données du https://clinicaltrials.gov, mis à jour le 2024-05-22)
| Numéro NCT | Recrutement | Conditions | Promoteur/Collaborateurs | Date de début | Phases |
|---|---|---|---|---|---|
| NCT05626920 | Recruiting | Inherited Retinal Dystrophy Primarily Involving Sensory Retina |
University of Washington |
December 2023 | Phase 1|Phase 2 |
| NCT04485130 | Terminated | Covid19 |
University of California San Francisco |
August 18 2021 | Phase 2 |
| NCT03151772 | Terminated | Glioblastoma |
Sahlgrenska University Hospital Sweden |
January 29 2018 | Early Phase 1 |
| NCT02309801 | Completed | Healthy |
Parc de Salut Mar|Ministerio de Sanidad Servicios Sociales e Igualdad |
July 2012 | Phase 1 |
Support technique
Tel: +1-832-582-8158 Ext:3
Si vous avez dautres questions, veuillez laisser un message.
